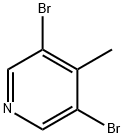
3,5-Dibromo-4-methylpyridine synthesis
- Product Name:3,5-Dibromo-4-methylpyridine
- CAS Number:3430-23-7
- Molecular formula:C6H5Br2N
- Molecular Weight:250.92
625-92-3
498 suppliers
$6.00/10g

74-88-4
350 suppliers
$15.00/10g

3430-23-7
205 suppliers
$5.00/250mg
Yield:-
Reaction Conditions:
Stage #1:3,5-dibromopyridine with n-butyllithium;diisopropylamine in tetrahydrofuran;hexane at -78 - -10; for 0.5 h;
Stage #2:methyl iodide in tetrahydrofuran;hexane at -78; for 2 h;
Steps:
2
A solution of n-butyllithium in hexane (1.6 N, 16 ml) is added dropwise, under argon, to a solution of diisopropylamine (3.6 ml, 1.02 equiv.) in anhydrous THF (145 ml) maintained at -10° C. The reaction mixture is cooled to -78° C. and then a solution of 3,5-dibromopyridine (5.92 g, 25 mmol) in THF (200 ml), cooled to -78° C., is added dropwise. The reaction mixture is stirred for 30 min and then methyl iodide (2.17 ml, 1.4 equiv.) is added dropwise. The stirring is maintained for 2 h at -78° C. A saturated aqueous NH4Cl solution (120 ml) is added. After evaporation of the solvents, the reaction mixture is extracted with EtOAc. The organic phase is washed with brine, dried over MgSO4 and evaporated. The yellow solid obtained is taken up with EtOAc. The suspension is filtered. The filtrate is evaporated and the residue is chromatographed on silica gel, elution being carried out with a petroleum ether/EtOAc (97.5/2.5) mixture. 1.67 g of product are obtained in the form of a white solid.
References:
SANOFI-AVENTIS US2007/185187, 2007, A1 Location in patent:Page/Page column 9

74-88-4
350 suppliers
$15.00/10g

3430-23-7
205 suppliers
$5.00/250mg

625-92-3
498 suppliers
$6.00/10g

74-88-4
350 suppliers
$15.00/10g

3430-23-7
205 suppliers
$5.00/250mg
125419-80-9
19 suppliers
inquiry