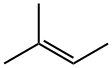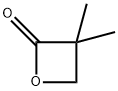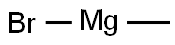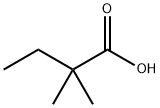
2,2-Dimethylbutyric acid synthesis
- Product Name:2,2-Dimethylbutyric acid
- CAS Number:595-37-9
- Molecular formula:C6H12O2
- Molecular Weight:116.16
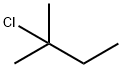
594-36-5
75 suppliers
$28.00/5g
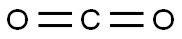
124-38-9
129 suppliers
$175.00/23402

595-37-9
283 suppliers
$15.00/5g
Yield:595-37-9 72%
Reaction Conditions:
Stage #1:2-methyl-2-butylchloride with magnesium in tetrahydrofuran;toluene at 50 - 90; for 2 h;
Stage #2:carbon dioxide in tetrahydrofuran;toluene at 35 - 45; for 1 h;
Stage #3: with hydrogenchloride in tetrahydrofuran;water;toluene for 4 h;Reflux;
Steps:
1.1; 1.2; 2.1-2.3; 3.1-3.2; 4.1; 5 Mechanism Preparation of tert-amyl chloride magnesium format reagent
Specifically, 27 g of magnesium turnings are added to the dried first reaction vessel, stirring is started and heating is started, and heating is stopped when heated to 50 ° C. When the temperature in the reaction vessel is washed to 60-70 ° C,A mixture of 2 g of tetrahydrofuran and 2 g of t-amyl chloride was added to initiate the reaction as an initiator. If a large amount of white smoke was generated in the reaction vessel and the temperature rose rapidly, the reaction was already initiated. When the process of temperature rise becomes slow and tends to stop, a mixture of 104.5 g of t-amyl chloride, 100 g of tetrahydrofuran, and 60 g of toluene is added dropwise at 80-90 ° C.After the addition of the mixed droplets, the mixture was incubated at 80-90 ° C for 2 h to obtain a reagent of tert-amylchloromagnesium format.Among them, tert-amyl chloride is obtained by the method provided in the specification of the present application.(2) Preparation of 2,2-dimethylbutyric acid using the following mechanismSpecifically, the t-amylhalo-halogen magnesium format reagent obtained in the step (1) is cooled to 35 ° C, and 88 g of carbon dioxide is introduced in a temperature range of 35-45 ° C, and the t-amyl magnesium halide format reagent is obtained by keeping the heat for 1 h. The addition reaction with carbon dioxide is carried out to obtain a 2,2-dimethylbutyrate reagent.Then, 150 g of hydrochloric acid and 300 g of water were added to the second reaction vessel, stirred and cooled, and the 2,2-dimethylbutyrate reagent obtained in the step (2) was added dropwise, and the mixture was added dropwise, and the mixture was heated to reflux for 4 hours, and cooled to room temperature. , a layered first reagent is obtained. The aqueous layer of the first reagent is removed, 500 ml of water and 60 g of the caustic soda are added to the oil layer of the first reagent, stirred for 30 min, and the layering is continued to obtain a second reagent. At this time, the 2,2-dimethylbutyric acid obtained by the hydrolysis has been It is extracted into the aqueous layer of the second reagent by alkali, and the oil layer of the second reagent is mainly organic, and can be recycled and reused.The aqueous layer of the second reagent is acidified with hydrochloric acid to pH=1 to obtain a layered third reagent, and the oil layer of the third reagent is subjected to vacuum distillation to collect a fraction of 94-95 ° C / 5 mmHg.2,2-Dimethylbutanoic acid prepared in Example of the present embodiment obtained were determined by gas chromatography, of greater than 99% purity, t-amyl alcohol to yield the product count, in 72% yield.
References:
Cheng Jiagang CN109608324, 2019, A Location in patent:Paragraph 0044; 0045; 0046; 0047; 0048; 0049; 0050-0074

813-67-2
7 suppliers
inquiry

595-37-9
283 suppliers
$15.00/5g