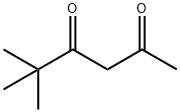
2,2-DIMETHYL-3,5-HEXANEDIONE synthesis
- Product Name:2,2-DIMETHYL-3,5-HEXANEDIONE
- CAS Number:7307-04-2
- Molecular formula:C8H14O2
- Molecular Weight:142.2

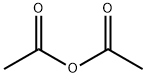
75-97-8
235 suppliers
$10.00/1g
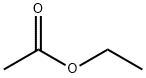
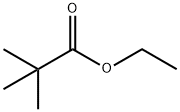
141-78-6
625 suppliers
$20.50/250ml

7307-04-2
66 suppliers
$38.00/5g
Yield: 54%
Reaction Conditions:
with sodium hydride in diethyl ether;mineral oil at 40 - 50;Inert atmosphere;
Steps:
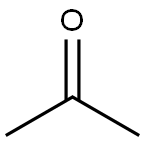
Pivaloylacetone (1b)
The solution of pinacolone (2g, 20mmol) in ether (2.4mL) was added to a stirred mixture of sodium hydride (0.96g, 40mmol, 60% in oil) in dry ethyl acetate (3.3mL, 40mmol) during 60min, so to maintain convenient rate of reaction. The temperature was kept at 40-50°C by gentle warming in an oil bath. After the addition of few mL of ketone solution, the addition was stopped and little alcohol was added. After all the pinacolone was added, the mixture was stirred at 40-50°C. Additional 20mL of ether was added during this time to maintain a fluid reaction mixture. The reaction mixture was cooled to rt and sufficient ether was added. Most of the unreactive sodium hydride was destroyed by the addition of ethanol and stirring for about 20min. The mixture was then cooled to rt and a mixture of ice water and slight excess of dil. HCl was added keeping the temperature below 20°C during neutralization. Stirring was continued until all solid was dissolved. The ether phase was separated and aqueous phase was extracted with ether. The combined ether extract was washed with NaHCO3 solution then with water. The solvent was removed and residue was purified by bulb-to-bulb distillation (60°Cat 10mm Hg) to obtain 1b as colorless liquid (2.61g, 54%).
References:
Saha, Debajyoti;Ghosh, Raju;Dutta, Ranjan;Mandal, Achintya Kumar;Sarkar, Amitabha [Journal of Organometallic Chemistry,2015,vol. 776,p. 89 - 97]
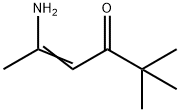
33663-62-6
0 suppliers
inquiry

7307-04-2
66 suppliers
$38.00/5g

75-97-8
235 suppliers
$10.00/1g

7307-04-2
66 suppliers
$38.00/5g