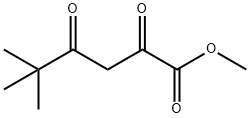
methyl 5,5-dimethyl-2,4-dioxohexanoate synthesis
- Product Name:methyl 5,5-dimethyl-2,4-dioxohexanoate
- CAS Number:42957-17-5
- Molecular formula:C9H14O4
- Molecular Weight:186.21

75-97-8
235 suppliers
$10.00/1g

7664-93-9
6 suppliers
$23.30/1090731000

95-92-1
418 suppliers
$5.00/5G

42957-17-5
7 suppliers
inquiry
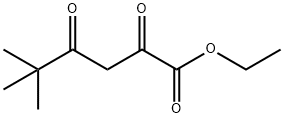
13395-36-3
94 suppliers
$13.43/250mgs:
Yield:13395-36-3 56.7% and 9.5%
Reaction Conditions:
with sodium in methanol;water;
Steps:
1 (for comparison)
EXAMPLE 1 (for comparison) 23 g of sodium are dissolved in 300 ml of absolute methanol according to the instructions in J. Am. Chem. Soc. 67, 1508 (1945). A mixture of 1 mol of oxalic acid diethyl ester and 1 mol of pinacolin is then added dropwise to the sodium methylate solution, which is kept at room temperature by cooling, in the course of 1 hour and with exclusion of atmospheric moisture. The mixture is stirred for a further 6 hours at room temperature and then left to stand overnight at room temperature. An ice-cold solution of 30 g of concentrated sulphuric acid in 200 ml of water is then added to the ice-cooled reaction batch. The mixture is stirred for 10 minutes, poured into 1 liter of water and then extracted with three 100 ml portions of benzene. The combined benzene extracts are washed twice with 100 ml of water each time and then separated by distillation. 127 g of reactions product which, according to analysis by gas chromatography, contains 83% by weight of pivaloylpyruvic acid methyl ester and 15% by weight of pivaloylpyruvic acid ethyl ester are obtained. The contents of methyl ester and ethyl ester in the 127 g amount of reaction product correspond to 56.7% and 9.5% of the theoretical yield.
References:
US4739104,1988,A

75-97-8
235 suppliers
$10.00/1g
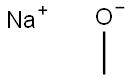
124-41-4
692 suppliers
$12.00/25g

95-92-1
418 suppliers
$5.00/5G

42957-17-5
7 suppliers
inquiry
