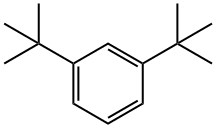
1,3-DI-TERT-BUTYLBENZENE synthesis
- Product Name:1,3-DI-TERT-BUTYLBENZENE
- CAS Number:1014-60-4
- Molecular formula:C14H22
- Molecular Weight:190.32
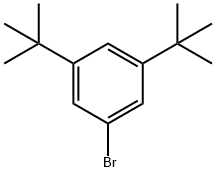
22385-77-9
299 suppliers
$5.00/1g

1014-60-4
116 suppliers
$53.00/1g
Yield:1014-60-4 67 %Chromat.
Reaction Conditions:
with C33H30F3N2NiO2P;tert-butylmagnesium chloride in tetrahydrofuran;diethyl ether at 70; for 24 h;Schlenk technique;Inert atmosphere;chemoselective reaction;
Steps:
General procedure for the catalytic dehalogenation
General procedure: A Schlenk flask was charged with an appropriate amount of complex 6 (0.05mmol, 5.0mol%) and the corresponding bromo, chloro, fluoro or iodo arene (1.0mmol). The flask was cycled with nitrogen and vacuum. Afterwards THF (2.0mL) and a diethylether solution of tert-butyl-magnesiumchloride (0.75mL, 1.5mmol, 2.0M in diethylether) were added. The flask was sealed and heated at 70°C for 24h or stirred at room temperature. After that time, the mixture was cooled and a NH4Cl solution was added. The mixture was extracted with dichloromethane and the organic layer was dried with Na2SO4. n-Dodecane was added and an aliquot was taken for GC-MS analysis. The coupling products were confirmed by GC-MS and NMR analysis using n-dodecane as internal standard. The analytical properties of the products are in agreement with literature data.
References:
Weidauer, Maik;Irran, Elisabeth;Someya, Chika I.;Haberberger, Michael;Enthaler, Stephan [Journal of Organometallic Chemistry,2013,vol. 729,p. 53 - 59] Location in patent:supporting information
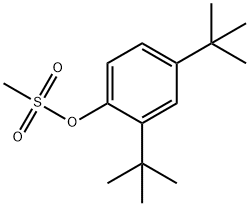
1071592-55-6
1 suppliers
inquiry

1014-60-4
116 suppliers
$53.00/1g
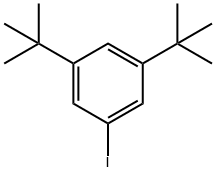
37055-53-1
31 suppliers
inquiry

1014-60-4
116 suppliers
$53.00/1g
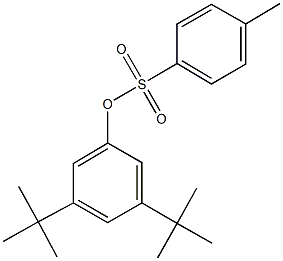
93131-79-4
2 suppliers
inquiry

1014-60-4
116 suppliers
$53.00/1g

71-43-2
694 suppliers
$11.19/25ML
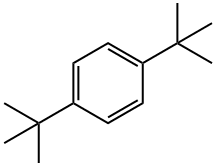
1012-72-2
134 suppliers
$9.00/1g

1014-60-4
116 suppliers
$53.00/1g