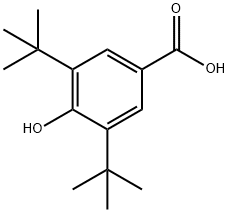
3,5-Di-tert-butyl-4-hydroxybenzoic acid synthesis
- Product Name:3,5-Di-tert-butyl-4-hydroxybenzoic acid
- CAS Number:1421-49-4
- Molecular formula:C15H22O3
- Molecular Weight:250.33

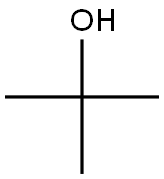
128-39-2
345 suppliers
$5.00/25g
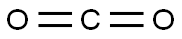
124-38-9
128 suppliers
$214.00/14L

1421-49-4
186 suppliers
$9.00/10g
Yield:1421-49-4 90.2%
Reaction Conditions:
Stage #1: 2,6-di-tert-butylphenolwith sodium methylate in methanol at 180; for 2 h;Simultaneous removal of methanol by distillation;
Stage #2: carbon dioxide at 200; under 4413.43 Torr; for 2 h;
Stage #3: with sulfuric acid in water; pH=3.8;Product distribution / selectivity;
Steps:
1; 2
Example 1 1207 g (5.85 moles) of DTBP comprising 0.01 % by weight of TTBBP, which was obtained in referential example, and 86.8g (0.45 moles) of 28% solution of sodium methoxide in methanol were fed in an 2L stainless-steel vessel equipped with a magnetic stirrer, a thermometer, pressure gauge and alcohol-separator. The reaction mixture was heated to 180°C under nitrogen gas flow and reacted at the temperature for 2 hrs. At the same time, methanol was distilled off. Thus obtained slurry of sodium salt of DTBP was heated to 200°C. The nitrogen gas in the vessel was replaced with carbon dioxide gas and carboxylation reaction was carried out under the CO2 pressure of 6 kgf/cm2(G) with stirring for 2 hrs. After the reaction was completed, the reaction mixture was cooled to 90°C and 1200 g of water was added thereto. The obtained mixture was separated into the aqueous and organic phases at 85 °C. To thus obtained aqueous phase, 73% aqueous sulfuric acid was added and the pH was adjusted to 3.8 to precipitate 3,5-di-tert-butyl-4-hydroxybenzoic acid. The precipitated crystal was filtrated, washed with water and dried. As a result, 101.6 g of 3,5-di-tert-butyl-4-hydroxybenzoic acid was obtained. The yield to the fed amount of sodium methoxide was 90.2%; Example 2 The organic phase obtained in the 6th cycle in Comparative Example 2, i.e. 1150g of DTBP containing 1.68% by weight of TTBBP was used. The organic phase was purified in the same manner as described in Referential Example and 920 g of DTBP containing 0.03% of TTBBP was obtained. Thus purified organic phase was added with 287 g of DTBP containing 0.01 % by weight of TTBBP to give 1207 g of DTBP containing 0.03% by weight of TTBBP. 3,5-di-tert-buthyl-4-hydroxy benzoic acid was prepared in the same manner as Example 1 using thus obtained DTBP for the starting DTBP. After the carboxylation reaction was completed, water was added to the mixture and then, the mixture was separated into the water and organic phases. To the water phase, 73% aqueous sulfuric acid was added and the pH was adjusted to 3.8 to precipitate 3,5-di-tert-butyl-4-hydroxybenzoic acid. The precipitated crystal was filtrated, washed with water and dried. As a result, 105 g of 3,5-di-tert-butyl-4-hydroxybenzoic acid was obtained. The yield to the fed amount of sodium methoxide was 93.2 %.
References:
EP1600436,2005,A1 Location in patent:Page/Page column 5-6; 6-7
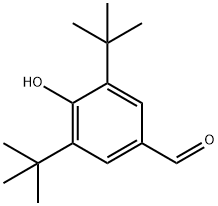
1620-98-0
253 suppliers
$6.00/5g

1421-49-4
186 suppliers
$9.00/10g
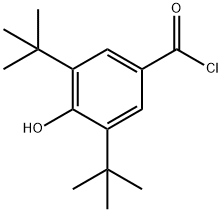
40056-43-7
0 suppliers
inquiry

1421-49-4
186 suppliers
$9.00/10g
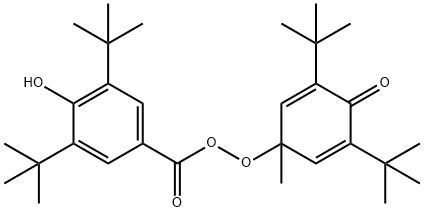
69901-39-9
0 suppliers
inquiry
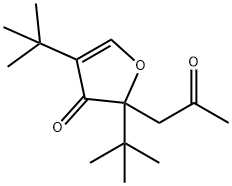
66483-14-5
0 suppliers
inquiry

719-22-2
171 suppliers
$14.00/5g
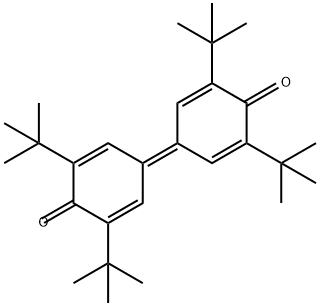
2455-14-3
121 suppliers
$6.00/1g

1421-49-4
186 suppliers
$9.00/10g