- Terbinafine Hydrochloride
-

- $0.00 / 1Kg/Bag
-
2024-12-20
- CAS:78628-80-5
- Min. Order: 1Kg/Bag
- Purity: 99% up, High Density
- Supply Ability: 20 tons
|
| Product Name: | Terbinafine Hydrochloride | | Synonyms: | Terbinafine hydrochloride, >=98%;Terbinafinehydrochloride(GMP);terbinafine hydrochloride, N,6,6-Trimethyl-N-(naphthalen-1-ylmethyl)hept-2-en-4-yn-1-amine hydrochloride;1-Naphthalenemethanamine, N-[(2E)-6,6-dimethyl-2-hepten-4-yn-1-yl]-N-methyl-, hydrochloride (1:1);Terbinafine Hydrochioride Tablets;n-(6,6-dimethyl-2-hepten-4-ynyl)-n-methyl-1-naphthalenemethanamin(e)-1-naphthalenemethanaminmon;TRANS-N-(6,6-DIMETHYL-2-HEPTEN-4-YNYL)-N-METHYL-1-NAPHTHYLMETHYLAMINE HYDROCHLORIDE;(e)-n-(6,6-dimethyl-2-hepten-4-ynyl)-n-methyl-1-naphthalenemethanamine monohydrochloride | | CAS: | 78628-80-5 | | MF: | C21H26ClN | | MW: | 327.89 | | EINECS: | 616-640-4 | | Product Categories: | Acetylenes;Antifungals for Research and Experimental Use;Biochemistry;Functionalized Acetylenes;Terbinafine;Antifungal;APIs;Amines;Aromatics;Intermediates & Fine Chemicals;Pharmaceuticals;LAMISIL;API;78628-80-5 | | Mol File: | 78628-80-5.mol | 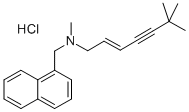 |
| | Terbinafine Hydrochloride Chemical Properties |
| Melting point | 204-208°C | | storage temp. | 15-25°C | | solubility | methanol: soluble50mg/mL | | form | powder | | color | white | | Merck | 14,9156 | | BCS Class | 1 | | InChI | InChI=1S/C21H25N.ClH/c1-21(2,3)15-8-5-9-16-22(4)17-19-13-10-12-18-11-6-7-14-20(18)19;/h5-7,9-14H,16-17H2,1-4H3;1H/b9-5+; | | InChIKey | BWMISRWJRUSYEX-SZKNIZGXSA-N | | SMILES | C12C=CC=CC1=CC=CC=2CN(C)C/C=C/C#CC(C)(C)C.Cl | | CAS DataBase Reference | 78628-80-5(CAS DataBase Reference) |
| Hazard Codes | Xi,N | | Risk Statements | 36/37/38-50/53 | | Safety Statements | 26-36/37/39-61-60 | | RIDADR | UN 3077 9/PG 3 | | WGK Germany | 3 | | RTECS | QJ8600100 | | HS Code | 29214990 | | Toxicity | LD50 in mice, rats (mg/kg): 4000, 4000 orally; 393, 213 i.v. (Ganzinger) |
| | Terbinafine Hydrochloride Usage And Synthesis |
| Dermatologist Broad-spectrum Antifungal Drugs | Terbinafine hydrochloride is a kind of broad-spectrum dermatologist allyl amine antifungal drugs. It is developed by Swiss Novartis in the 1980 s, and appeared in the market of UK for the first time in 1991. Approved by FDA of the United States for OTC drugs in 1996, and appeared in the market of the United States in the same year. At present, the drug is been sold in more than 90 countries of the word. It can specificity trouble the late biological decomposition of fungus sterol, selectively inhibit the activity of fungal squalene ring oxidase, and inhibit the squalene epoxidation in the formation of ungal cell membrane, thus to kill or inhibit the active of the fungus. Suitable for treatment of candidiasis skin, such as tinea manuum, tinea, tinea, ringworm of the body, tinea versicolor, it is also the best medicine for the treatment of onychomycosis.
Terbinafine hydrochloride entered the the first batch of country announced OTC directory in 2000. This product belongs to antifungal drugs. It has strong effect on shallow fungal infection, and can cure most of the fungal skin diseases through external use. | | Pharmacokinetics | According to reports in the literatures, after 250 mg of Terbinafine hydrochloride been taken orally, it reaches peak plasma concentration of 0.97 m ug/ml within 2 hours. The absorption half-life is 0.8 hours, spread half-life is 4.6 hours, the degree of biological application is slightly affected by eating, but not used for dose adjustments. The combination rate of drugs and plasma protein is 99%, and can quickly disperse and concentrate in the lipophilic corneous layer through the dermis. Terbinafine hydrochloride can be spread in the skin, therefore, a very high concentration can be reached in the hair follicle, hair and skin layers of fat, and it can enter in the deck in the last few weeks of healing. The metabolites after the bioconversion have no antifungal activity, then discharged through urine. The half-life is 17 hours, no accumulation in the body, and its steady blood drug concentration is not affected by age. But the alleviation rate can reduce for people with liver and kidney disorders, thus triggers blood drug concentration decreasing. | | Usage and Dosage | Local external use: apply adequate amount to the affected area and its surrounding, 1-2 times a day. Ringworm of the body, 2-4 weeks, tinea of feet and hands, tinea versicolor, 4 to 6 weeks.
Oral: 0.25 grams each time for adult, once per day, period of treatment is as follows:
Skin infection treatment: tinea of feet and hands [toe (means) and plantar]: 2~6 weeks; Ringworm of the body, tinea, 2~4 weeks.
Skin candidiasis: 2~4 weeks. normal skin appearance and loss of infection can only be visible in a few weeks for curing.
Hair and scalp infection treatment: tinea capitis: 4 weeks, tinea capitis most happen in children.
Onychomycosis: The most treatment course of patients is 6 weeks to three months. Young patients with normal growth can shorten the treatment course, and less than 3 months of treatment may be enough in addition to the thumb (foot) armour.
In other cases, the treatment is usually 3 months. Some patients, especially whose thumb (toe) infected, may take 6 months or longer. The treatment may take more than 3 months if the nail grew slowly in the first week. In healing of mycology and stopping for a few months after treatment, the continuous improvement of nail appearance then completely normal can be seen, this is because that the healthy tissue growth needs time. | | Description | Terbinafine hydrochloride is the first orally active allylamine antifungal with 30-fold
greater antifungal activity than naftifine. The compound is indicated for the treatment
of ringworm and fungal nail infections. Terbinatine hydrochloride acts on a single
fungal enzyme, squalene epoxidase, interfering with the biosynthesis of ergosterols in
cell membranes. Unlike other antifungal agents, it does not inhibit cytochrome P450
enzymes. | | Chemical Properties | Terbinafine hydrochloride is an off-white crystalline material that is soluble in polar organic solventssuch as methanol, ethanol, and methylene chloride but isonly slightly soluble in water. The highly lipophilic freebase is insoluble in water. | | Originator | Sandoz (Switzerland) | | Uses | An orally active, antimycotic allylamine related to Naftifine. A specfic inhibitor of squalene epoxidase, a key enzyme in fungal ergosterol biosynthesis. Antifungal. | | Uses | Terbinafine hydrochloride may be used as a pharmaceutical reference standard for the determination of the analyte in drug dosage forms by UV spectrophotometry and high-performance thin layer chromatography (HPTLC). | | Definition | ChEBI: A hydrochloride obtained by reaction of terbinafine with one molar equivalent of hydrogen chloride. | | Indications | Terbinafine Hydrochloride is a synthetic allylamine antifungal
agent, structurally related to naftifine. It inhibits fungal sterol biosynthesis
by inhibiting the enzyme squalene 2,3-epoxidase. The deficiency of
ergosterol and concomitant accumulation of squalene within the fungal
cell results in cell death. It can be fungicidal or fungistatic, depending on
the concentration used, and is effective against dermatophytes, Aspergillus,
blastomycosis, and histoplasmosis. It is only minimally effective against
Candida. | | Manufacturing Process | To prepare terbinafine hydrochloride, N-methyl-1-naphthalene methylamine hydrochloride (29 kg) was added to a reactor containing dimethylformamide (70.4 liters) and water (11 liters). The mixture was stirred for 15 minutes until fully dissolved. Sodium carbonate (11 kg) was added and the reaction mixture was cooled to 13 °C. Then, 1-chloro-6,6-dimethyl-2-heptene-4-yne (22 kg) was slowly added at a temperature range of 11 to 14 °C. The mixture was stirred for 60 minutes at 12-14 °C, then heated to 55 °C and maintained at 60 °C for 5 hours. Thin layer chromatography was used to confirm completion of the reaction. The mixture was cooled to room temperature and water (99 liters) was added. The mixture was extracted three times with dichloromethane (75 liters in total). The organic layer was washed twice with water (88 liters in total) and additional water (18 liters) was added to the organic layer, which was then cooled to 13 °C. The pH of the reaction mass was adjusted to 0.2 by adding 36% aqueous hydrochloric acid (15 liters) and stirring for 30 minutes. The organic layer was separated and washed three times with water (267 liters in total). The final organic layer was transferred to a reactor and the solvent was distilled below 45 °C. Petroleum ether (11.8 liters) was added and the solvent was completely distilled below 50 °C. An additional amount of petroleum ether (68 liters) was added and the mixture was heated to reflux. After 30 minutes of stirring at reflux, the mixture was cooled to 50 °C. The solid formed was allowed to settle for 60 minutes and the top petroleum ether layer was decanted. This decantation process was repeated twice more. Finally, 44 liters of petroleum ether was added, heated to reflux for 30 minutes, and then cooled to 25 °C. The mixture was stirred for 60 minutes at 20-25 °C, centrifuged to collect the solid, and washed twice with petroleum ether (2x16 liters). The solid was then spin-dried for about 60 minutes to obtain 29.3 kg of crude terbinafine hydrochloride as a semi-dry solid.
WO2007096904A2 Improved process for the preparation of terbinafine hydrochloride and novel crystalline form of terbinafine | | Brand name | Lamisil | | Therapeutic Function | Antifungal | | General Description | Terbinafine Hydrochloride is a synthetic allylamine derivative structurally related to naftifine, antifungal Terbinafine Hydrochloride blocks ergosterol biosynthesis by inhibition of squalene epoxidase, part of the sterol synthesis pathway for the fungal cell membrane. | | Biological Activity | Terbinafine is a broad-spectrum antifungal agent that has activity against T. rubrum, T. metagrophytes, T. verrucosum, E. floccosum, M. canis, A. fumigatus, and S. schenckii (MIC50s = 0.003-0.8 μg/ml). It selectively inhibits C. albicans squalene epoxidase over rat liver epoxidase (IC50s = 0.03 and 77 μM, respectively). Terbinafine (90-120 μM) induces cell cycle arrest at the G0/G1 phase in COLO 205 tumor cells and human umbilical vein endothelial cells (HUVECs). Formulations containing terbinafine have been used in the treatment of nail and skin fungal infections. | | Biochem/physiol Actions | Mode of Action: Inhibits squalene epoxidase, preventing biosynthesis of ergosterol.
Antimicrobial spectrum: Antifungal and antimycotic. Fungicidal against dermatopytes and some yeasts; fungistatic against Candida albicans. | | Clinical Use | Terbinafine hydrochloride (Lamisil) is available for
topical and systemic use (oral tablet) in the treatment of
dermatophyte skin and nail infections. Terbinafine also
exhibits in vitro activity against filamentous and dimorphic
fungi, but its clinical utility in treating infections
with these organisms has not yet been established. It is
used most commonly in the treatment of onychomycosis;
in this setting, terbinafine is superior to griseofulvin
and at least equivalent to itraconazole.When given systemically,
terbinafine is 99% protein bound and accumulates
in fat, skin, and nails, persisting for weeks.
Cerebrospinal fluid penetration is less than 10%.
Dosage reductions are required with renal or hepatic
insufficiency. Although terbinafine has little effect on
hepatic cytochrome P450 enzyme systems, it does minimally
enhance cyclosporine clearance. Oral terbinafine
is generally well tolerated but occasionally causes gastric
distress and liver enzyme elevation. | | Side effects | Adverse reactions include lens and retinal disturbances (red/green visual
perception), metallic taste disturbance that may last up to 4 weeks after
medication discontinuation, hepatoxicity, and pancytopenia. Five percent of
patients will experience delayed gastric emptying with symptoms of nausea,
fullness, and/or dyspepsia. Terbinafine does not appear to have any effect on
the cytochrome P-450 systems (Check baseline CBC and LFTs; repeat if taken
for >6 weeks). | | Synthesis | Terbinafine is produced from olefin metathesis of 1,3-dichloropropene and neohexene followed by reaction with N-methyl-1-naphthalenemethanamine. | | Veterinary Drugs and Treatments | Terbinafine may be useful for treating dermatophytic and other fungal infections in dogs and cats. It may also be useful for treating birds for systemic mycotic (e.g., aspergillosis) infections. |
| | Terbinafine Hydrochloride Preparation Products And Raw materials |
|