Trovafloxacin Mesylate manufacturers
|
| | Trovafloxacin Mesylate Basic information |
| Product Name: | Trovafloxacin Mesylate | | Synonyms: | 7-[(1α,5α,6α)-6-AMino-3-azabicyclo[3.1.0]hex-3-yl]-1-(2,4-difluorophenyl)-6-fluoro-1,4-dihydro-4-oxo-1,8-naphthyridine-3-carboxylic Acid Methanesulfonate;CP 99219-27;7-[(1S,5R)-6-amino-3-azabicyclo[3.1.0]hex-3-yl]-1-(2,4-difluorophenyl)-6-fluoro-4-oxo-1,8-naphthyridine-3-carboxylic acid;Trovafloxacin monomethanesulfonate;Trovan;Unii-0p1lko80wn;7-[(1α,5α,6α)-6-Amino-3-azabicyclo[3.1.0]hex-3-yl]-1-(2,4-difluorophenyl)-6-fluoro-1,4-dihydro-4-oxo-1,8-naphthyridine-3-carboxylicacidmesylate;Trovafloxacin Methanesulfonate | | CAS: | 147059-75-4 | | MF: | C21H19F3N4O6S | | MW: | 512.46 | | EINECS: | | | Product Categories: | Amines;Chiral Reagents;Heterocycles;Intermediates & Fine Chemicals;Pfizer compounds;Pharmaceuticals | | Mol File: | 147059-75-4.mol | 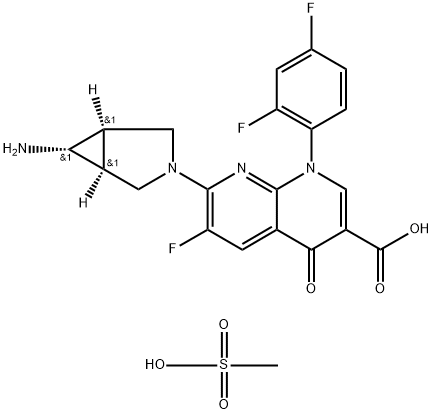 |
| | Trovafloxacin Mesylate Chemical Properties |
| Melting point | >250°C (dec.) | | storage temp. | room temp | | solubility | DMSO: >10mg/mL | | form | powder | | color | white to off-white |
| Hazard Codes | C | | Risk Statements | 63-34 | | Safety Statements | 26-36-45 | | RIDADR | UN 1759 8 / PGIII | | WGK Germany | 3 | | RTECS | QN2791250 | | Toxicity | women,LDLo,oral,2mg/kg (2mg/kg),SKIN AND APPENDAGES (SKIN): "DERMATITIS, OTHER: AFTER SYSTEMIC EXPOSURE",Archives of Internal Medicine. Vol. 159, Pg. 2225, 1999. |
| | Trovafloxacin Mesylate Usage And Synthesis |
| Description | Trovafloxacin is a broad spectrum antibiotic that inhibits the uncoiling of supercoiled DNA in bacteria by blocking the activity of topoisomerase IV (IC50 = 3.02 μg/ml) and DNA gyrase (IC50 = 7.13 μg/ml). | | Chemical Properties | White Solid | | Originator | Trovan,Pfizer,USA | | Uses | Trovafloxacin mesylate has been used as a test compound in toxicity assay to assess its toxicity in 2D hepatocyte cultures. It has also been used to manipulate the mechanism of apoptotic cell disassembly during apoptosis. | | Uses | Fluorinated quinolone antibacterial. Trovafloxacin mesylate blocks the activity of DNA gyrase and topoisomerase IV, enzymes essential in the repliction, transcription, and repair of bacterial DNA. | | Definition | ChEBI: A methanesulfonate (mesylate) salt prepared from equimolar amounts of trovafloxacin and methanesulfonic acid. A broad-spectrum antibiotic that was withdrawn from the market due to risk of liver failure. | | Manufacturing Process | N-Benzylmaleimide (500 g, 2.67 mole), 90% bromonitromethane (831 g, 5.34
mole), powdered molecular sieves 200 mesh (2020 g) and toluene (12 dm3)
were stirred under nitrogen at -10°C. 1,2-Dimethyl-1,4,5,6-
tetrahydropyrimidine (616 g, 5.49 mole) was added slowly over about 3 h
maintaining the reaction temperature at <-8°C throughout the addition. After
completion of the addition, the reaction mixture was stirred for 1.5 h at 25°C,
filtered under a nitrogen atmosphere in a sealed pressure filter to remove
sieves and resulting tar, and the sieves were washed with toluene (2 L). The
combined filtrates were washed with 2 N dilute hydrochloric acid (3 times 750
cm3), treated with carbon (50 g) at 70°C, 1 h filtered, concentrated, and
triturated with 2-propanol (about 4 dm3) to obtain crystals of the (1α,5α,6α)-
3-N-benzyl-6-nitro-2,4-dioxo-3-azabicyclo[3.1.0]hexane (223 g, 34%) melting
point 116°-118°C.
Tetrahydrofuran (350 cm3), sodium borohydride (14.1 g) and (1α,5α,6α)-3-N_x0002_benzyl-6-nitro-2,4-dioxo-3-azabicyclo[3.1.0]hexane (35.0 g, mmol) obtained
above were stirred under nitrogen for 0.25 h and then treated dropwise with
boron trifluoride-THF complex containing 21.5% BF3 (44.9 cm3) so that the
exotherm was controlled to <40°C. After addition was completed, the reaction
mixture was stirred for 3 h at 40°C, quenched slowly with water/THF 1:1 (70
cm3) to avoid excessive foaming, and stirred for 0.5 h at 50°C to ensure that
the quench of unreacted diborane generated in situ was completed. The
quench formed a salt slurry which was filtered and washed with THF (140
cm3); the combined filtrate was partially concentrated, diluted with water (350
cm3) and further concentrated to remove most of the THF, and extracted with
ethyl acetate (140 cm3). The resulting ethyl acetate solution was concentrated
to afford the (1α,5α,6α)-3-N-benzyl-6-nitro-3-azabicyclo[3.1.0]hexane as a
clear oil (30.6 g, 97%).
(1α,5α,6α)-3-N-Benzyl-6-nitro-3-azabicyclo[3.1.0]hexane (30.9 g, 142 mmol)
obtained above, 2-propanol (310 cm3), water (30 cm3), and 10% Pd on
carbon, 50% water content (12.3 g) were hydrogenated at 50 psi and 50°C
for 18-24 h in a Parr shaker. The Pd catalyst was filtered off, and the resulting
pale yellow filtrate was azeotropically distilled at constant volume to remove
water. The resulting solution was treated with triethylamine (46 g, 456 mmol)
and heated to reflux. Benzaldehyde (15.0 g, 141 mmol) was added dropwise
over 15 min. The reaction mixture was heated at reflux for 4 h to form
(1α,5α,6α)-6-benzylidenylamino-3-azabicyclo[3.1.0]hexane in situ. The
resulting orange solution was cooled to 40°-50°C, and ethyl 7-chloro-1-(2,4-difluorophenyl)-6-fluoro-1,4-dihydro-4-oxo-1,8-naphthyridine-3-carboxylic acid
(42.45 g, 111 mmol; see United Kingdom Patent Publication No. GB
2,191,776) and triethylamine (13.1 g, 130 mmol) were added. The resulting
slurry was heated at reflux for 16-18 h, cooled to 20°C and stirred for 5 h.
The slurry was filtered, and the compound was isolated as a white solid
(75.5% yield based on (1α,5α,6α)-3-N-benzyl-6-nitro-2,4-dioxo-3-
azabicyclo[3.1.0]hexane; 96.6% based on ethyl 7-chloro-1-(2,4-
difluorophenyl)-6-fluoro-1,4-dihydro-4-oxo-1,8-naphthyridine-3-carboxylic
acid). The ethyl (1α,5α,6α)-7-(6-benzylidenylamino-3-azabicyclo[3.1.0]hex-
3yl)-1-(2,4-difluorophenyl)-6-fluoro-1,4-dihydro-4-oxo-1,8-naphthyridine-3-
carboxylate was recrystallized from acetonitrile, melting point 148°-155°C
decomp.
Tetrahydrofuran (250 cm3), ethyl (1α,5α,6α)-7-(6-benzylidenylamino-3-
azabicyclo[3.1.0]hex-3-yl)-1-(2,4-difluorophenyl)-6-fluoro-1,4-dihydro-4-oxo-
1,8-naphthyridine-3-carboxylate (25.05 g, 47 mmol) obtained above, and
water (250 cm3) were treated with 97% methanesulfonic acid (13.3 g, 138
mmol) and heated to reflux for 24 h. The resulting solution was cooled to
45°C, treated with activated carbon (2.5 g) for 1 h and filtered. The resulting
filtrate was concentrated under vacuum to approximately 25% of its original
volume to provide a white crystal slurry, cooled to 15°-25°C, granulated for 4
h and filtered to yield the trovafloxacin methanesulfonate salt (mesylate)
(16.86 g, 70.0%). Melting point 253°-256°C decomp. | | Brand name | Trovan (Pfizer). | | Therapeutic Function | Antibacterial | | Biological Activity | Fluoroquinolone antibiotic. Inhibits bacterial DNA topoisomerase IV and DNA gyrase and forms a stable quinolone-DNA complex with these enzymes which reversibly inhibits DNA synthesis. Displays potent activity against gram-positive and gram-negative bacteria. | | Biochem/physiol Actions | Trovafloxacin mesylate acts as a pannexin1 (Panx1) inhibitor. | | storage | Desiccate at RT |
| | Trovafloxacin Mesylate Preparation Products And Raw materials |
|