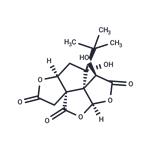
- Bilobalide
-

- $0.00 / 10mg
-
2024-12-20
- CAS:33570-04-6
- Min. Order: 10mg
- Purity: ≥98%HPLC
- Supply Ability: No limitation
- Bilobalide
-
- $50.00 / 20mg
-
2024-11-19
- CAS:33570-04-6
- Min. Order:
- Purity: 99.91%
- Supply Ability: 10g
- Bilobalide
-

- $0.00 / 1kg
-
2024-05-30
- CAS:33570-04-6
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 50000kg
|
| | Bilobalide Basic information |
| Product Name: | Bilobalide | | Synonyms: | BILOBALID;4H,5aH,9H-Furo[2,3-b]furo[3',2':2,3]cyclopenta[1,2-c]furan-2,4,7(3H,8H)-trione, 9-(1,1-dimethylethyl)-10,10a-dihydro-8,9-dihydroxy-, (3aS,5aR,8R,8aS,9R,10aS)-;(-)-BILOBALIDE;BILOBALIDE;5,3'-DIHYDROXY-3,6,7,4'-TETRAMETHOXYFLAVONE;4H,5AH,9H-FURO[2,3-B]FURO[3',2':2,3]CYCLOPENTA[1,2-C]FURAN-2,4,7(3H,8H)-TRIONE,9-(1,1-DIMETHYLETHYL)-10,10A-DIHYDRO-8,9-DIHYDROXY-, [5AR-(3AS*,5A,8,8AS*,9,10A)]-;3',5-DIHYDROXY-3,4',6,7-TETRAMETHOXY FLAVONE;VITEXICARPIM | | CAS: | 33570-04-6 | | MF: | C15H18O8 | | MW: | 326.3 | | EINECS: | 608-892-9 | | Product Categories: | Inhibitors;chemical reagent;pharmaceutical intermediate;phytochemical;reference standards from Chinese medicinal herbs (TCM).;standardized herbal extract;Heterocycles;Biochemicals Found in Plants;Isoprenoid/terpenoid;Nutrition Research | | Mol File: | 33570-04-6.mol | 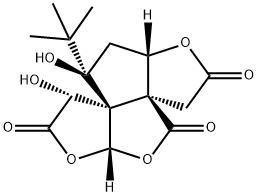 |
| | Bilobalide Chemical Properties |
| Melting point | >300° | | alpha | 57820 -64.3° (c = 2 in acetone) | | Boiling point | 651.7±55.0 °C(Predicted) | | density | 1.56±0.1 g/cm3(Predicted) | | storage temp. | −20°C | | solubility | Acetone (Slightly), Methanol (Slightly) | | pka | 11.74±0.40(Predicted) | | form | Solid | | color | White to Off-White | | Merck | 14,1220 | | Stability: | Hygroscopic | | LogP | -0.450 (est) | | CAS DataBase Reference | 33570-04-6(CAS DataBase Reference) |
| Safety Statements | 24/25 | | WGK Germany | 3 | | RTECS | LV1665000 | | F | 10 | | HS Code | 29322090 |
| | Bilobalide Usage And Synthesis |
| Description | Bilobalide is a sesquiterpene lactone which is found in extracts of G. biloba. It has been shown to protect against cerebral edema, decrease cortical infarct volume, and reduce cerebral ischemic damage. Bilobalide, at 10 μM, reduces the release of glycine and glutamate from hippocampal slices under ischemic conditions. It also activates the rat constitutive androstane receptor at 100 μM and increases the levels and activities of several cytochrome P450 isoforms in rat liver microsomes. | | Uses | Bilobalide provides protection against learning and memory impairment by reducing free radical injury and inhibiting neuronal apoptosis in the brain cortex and hippocampal CA1 region in induced vascular dementia rats. | | Uses | Reference Standard in the analysis of herbal medicinal products | | Definition | ChEBI: A terpenoid trilactone found in extracts of Ginkgo biloba. | | General Description | Produced and qualified by HWI pharma services GmbH.
Exact content by quantitative NMR can be found on the certificate. | | Enzyme inhibitor | This plant natural product (FW = 326.30 g/mol; CAS 33570-04-6) from the gingko tree (Ginkgo biloba) is a terpene trilactone that exhibits anticonvulsant properties. Bilobalide has multiple mechanisms of action (e.g., acting as a GABAA receptor antagonist (/1), preserving mitochondrial ATP synthesis, inhibiting staurosporine-induced apoptotic damage, suppressing hypoxia-induced membrane deterioration in the brain, and increasing the expression the mitochondrial DNA-encoded COX III subunit of cytochrome c oxidase and the ND1 subunit of NADH dehydrogenase). Bilobalide was later synthesized in E. J. Corey’s laboratory in the late 1980s. |
| | Bilobalide Preparation Products And Raw materials |
|