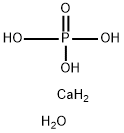
?? ???, ????, ????
|
|
?? ???, ????, ???? ??
- ???
- 109°C -H₂O
- ??
- 2.31
- ?? ??
- Inert atmosphere,Room Temperature
- ???
- ?? ???(96%)? ?? ?? ????. ????? ????? ???.
- ??? ??
- ??
- ??? ??
- ??? ??
- ??
- ???? ?? ??
- ???
- ?? ?? ?????. ?? ??, ??, ????? ?????. ???? ???
- ???
- ????. ?? ???? ????.
- InChI
- InChI=1S/Ca.H3O4P.H2O.2H/c;1-5(2,3)4;;;/h;(H3,1,2,3,4);1H2;;
- InChIKey
- XAAHAAMILDNBPS-UHFFFAOYSA-L
- SMILES
- P(O)(O)(O)=O.[Ca].O
- LogP
- -2.148 (est)
- CAS ??????
- 7789-77-7(CAS DataBase Reference)
??
- ?? ? ?? ??
- ?? ? ???? ?? (GHS)
?? ???, ????, ???? C??? ??, ??, ??
??? ??
white crystalline solid??
Dicalcium phosphate dihydrate is only slightly soluble at ordinary temperatures of mixing and holding doughs and batters. As a result, it does not release acidity for reaction with soda until late in the baking stage, when the temperature reaches 135 to 140°F. Since DCP·2H20 does not begin to react below 135°F, and the interior structure of a baked product begins to firm at about 160°F, a product that bakes rapidly may not provide sufficient time for complete release of all the C02. DCP·2H2 0, therefore, cannot be used in biscuits, pancakes or any baked product that is completely baked in less than 20 min.Dicalcium phosphate dihydrate is seldom used by itself in leavening systems but is usually combined with fasterreacting acidic phosphates. Its major applications are in cake mixes, frozen bread doughs, and other products requiring a half hour or more to complete baking. It has a low neutralizing value, and therefore more DCP·2H20 is required to neutralize a given amount of soda than for other phosphate-leavening acids.
?? ??
Calcium phosphates are usually manufactured by reacting very pure phosphoric acid with calcium hydroxide, Ca(OH)2 obtained from limestone, in stoichiometric ratio in aqueous suspension followed by drying at a temperature that will allow the correct hydration state to be achieved. After drying, the coarse-grade material is obtained by means of a classification unit; the fine particle-size material is obtained by milling.Pharmaceutical Applications
Dibasic calcium phosphate dihydrate is widely used in tablet formulations both as an excipient and as a source of calcium and phosphorus in nutritional supplements. It is one of the more widely used materials, particularly in the nutritional/health food sectors. It is also used in pharmaceutical products because of its compaction properties, and the good flow properties of the coarsegrade material. The predominant deformation mechanism of dibasic calcium phosphate coarse-grade is brittle fracture and this reduces the strain-rate sensitivity of the material, thus allowing easier transition from the laboratory to production scale. However, dibasic calcium phosphate dihydrate is abrasive and a lubricant is required for tableting, for example about 1% w/w of magnesium stearate or about 1% w/w of sodium stearyl fumarate is commonly used.Two main particle-size grades of dibasic calcium phosphate dihydrate are used in the pharmaceutical industry. The milled material is typically used in wet-granulated, roller-compacted or slugged formulations. The ‘unmilled’ or coarse-grade material is typically used in direct-compression formulations.
Dibasic calcium phosphate dihydrate is nonhygroscopic and stable at room temperature. However, under certain conditions of temperature and humidity, it can lose water of crystallization below 1008℃. This has implications for certain types of packaging and aqueous film coating since the loss of water of crystallization appears to be initiated by high humidity and by implication high moisture vapor concentrations in the vicinity of the dibasic calcium phosphate dihydrate particles.
Dibasic calcium phosphate dihydrate is also used in toothpaste and dentifrice formulations for its abrasive properties.
Safety
Dibasic calcium phosphate dihydrate is widely used in oral pharmaceutical products, food products, and toothpastes, and is generally regarded as a nontoxic and nonirritant material. However, oral ingestion of large quantities may cause abdominal discomfort.??
Dibasic calcium phosphate dihydrate is a nonhygroscopic, relatively stable material. However, under certain conditions the dihydrate can lose water of crystallization. This has implications for both storage of the bulk material and coating and packaging of tablets containing dibasic calcium phosphate dihydrate.The bulk material should be stored in a well-closed container in a cool, dry place.
Purification Methods
Crystallise it from a near-saturated solution in 50% aqueous reagent grade phosphoric acid at 100o by filtering through fritted glass and cooling to room temperature. The crystals are filtered off, and this process is repeated three times using fresh acid. For the final crystallisation the solution is cooled slowly with constant stirring to give thin plate crystals that are filtered off on a fritted glass funnel, washed free of acid with anhydrous acetone and dry in a vacuum desiccator [Egan et al.J Am Chem Soc 78 1811 1956].? ???
Dibasic calcium phosphate dihydrate should not be used to formulate tetracycline antibiotics. Dibasic calcium phosphate dihydrate has been reported to be incompatible with indomethacin, aspirin, aspartame, ampicillin, cephalexin, and erythromycin. The surface of dibasic calcium phosphate dihydrate is alkaline and consequently it should not be used with drugs that are sensitive to alkaline pH.Regulatory Status
GRAS listed. Accepted as a food additive in Europe. Included in the FDA Inactive Ingredients Database (oral capsules and tablets). Included in nonparenteral medicines licensed in Europe. Included in the Canadian List of Acceptable Non-medicinal Ingredients.?? ???, ????, ???? ?? ?? ? ???
???
???????
Phosphate rock powder
?? ??
????
Calcium chloride solution 36-40%, (1box=27kgs)
???
????(???)
?1????
??
ROCK PHOSPHATE
Calcite powder
Indicator
????(??)
?? ??
C.I. ?? ?? 9
??
phosphoric acid by wet process
??
????? ??
??
???, ???, ?
??
?????(??)
?? ??
?? ???, ????, ???? ?? ??
???( 396)?? ??
| ??? | ?? | ??? | ?? | ?? ? | ?? |
|---|---|---|---|---|---|
| Hebei Andu Technology Com.,Ltd | +86-86-17798073498 +8617798073498 |
admin@hbandu.com | China | 299 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 |
sales@hbmojin.com | China | 12835 | 58 |
| Wuhan Fortuna Chemical Co., Ltd | +86-027-59207850 |
info@fortunachem.com | China | 5986 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 |
sales1@chuanghaibio.com | China | 5893 | 58 |
| Hebei Kingfiner Technology Development Co.Ltd | +86-15532196582 +86-15373005021 |
lisa@kingfinertech.com | China | 2990 | 58 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 |
info@dakenam.com | China | 18754 | 58 |
| Jiangsu Kolod Food Ingredients Co.,Ltd. | +86-518-85110578 +8618805133257 |
sales3257@jskolod.com | China | 132 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21638 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +8618949832763 |
info@tnjchem.com | China | 2986 | 55 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 |
zheyansh@163.com | CHINA | 3619 | 58 |