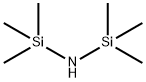
?????????
|
|
????????? ??
- ???
- -78 °C
- ?? ?
- 125 °C (lit.)
- ??
- 0.774 g/mL at 25 °C(lit.)
- ?? ??
- 4.6 (vs air)
- ???
- 20 hPa (20 °C)
- ???
- n
20/D 1.407(lit.)
- ???
- 57.2 °F
- ?? ??
- Store below +30°C.
- ???
- ???, ??, ?? ???, ?? ? ???????? ?? ?????.
- ?? ?? (pKa)
- 30(at 25℃)
- ??? ??
- ??
- Specific Gravity
- 0.774
- ??
- ???
- ??
- ???? ??
- pH ??
- 8.5
- ????
- 0.8-25.9%(V)
- ???
- ?? ??
- ??
- Moisture Sensitive
- Hydrolytic Sensitivity
- 7: reacts slowly with moisture/water
- Merck
- 14,4689
- BRN
- 635752
- InChIKey
- FFUAGWLWBBFQJT-UHFFFAOYSA-N
- LogP
- 0.23-1.19 at 20-25℃
- CAS ??????
- 999-97-3(CAS DataBase Reference)
??
- ?? ? ?? ??
- ?? ? ???? ?? (GHS)
| ??? ?? | F,C,Xn | ||
|---|---|---|---|
| ?? ???? ?? | 11-20/21/22-34-52/53 | ||
| ????? | 16-26-36/37/39-45-61 | ||
| ????(UN No.) | UN 3286 3/PG 2 | ||
| WGK ?? | 2 | ||
| RTECS ?? | JM9230000 | ||
| F ?????? | 21 | ||
| ?? ?? ?? | 325 °C | ||
| ?? ?? ?? | Flammable | ||
| TSCA | Yes | ||
| HS ?? | 2931 90 00 | ||
| ?? ?? | 3 | ||
| ???? | II | ||
| ?? ?? ??? | 999-97-3(Hazardous Substances Data) | ||
| ?? | LDLO i.p. in mice: 650 mg/kg (Petrarch Systems Inc. Product Information Sheets) | ||
| ???? ?? | KE-34695 |
????????? C??? ??, ??, ??
?? ??
?? ?? ? ?? ? ??. ?? ?? ? ???? ?? ?? ?? ??? NH3? ??. ????? ?? ?? ??? ?? ?? ?? ??? ?? ?? ??? ??? ??. ?? ???? ?? ? ??? NH3 ?? NH4Cl, trimethylchlorosilane? ?????. ????? NH3? ?? trimethylchlorosilane ????. N ??? ???? ?? ?? ?? ??? ????? ??? ? ??? ?? ?? ?????, ??? ?? ?? ??, ??? ? ??? ????? ?? ?? ?? ??, ?? ???? ??? ?? ??? ? ???? ??? ?? ???. ? ?? ?? ???? ??.??
HMDS ?? ?? ? ?? ? ? ?? ??? (? : amikacin, penicillin, cephalosporins, fluorouracil ? ???? ???? ??), ???? ?? ?? ????? ?? ?? ???? ?? ?????. ???? ?? ???, ?? ?? ??, ??? ? ??? ??? ?? ????? ??? ????? ?? ??? ? ??? ???? ??? ? ????.??, ?? ? ??
Hexamethydisilazane ??? ???? ???? ??? ??? ?? ? ??? ???????.??
Hexamethyldisilazane is a bulk organo silicon compound, being a quite useful silanizing agent. It is a reagent for the preparation of trimethylsilyl derivatives. It can also be used to dehydrate cells of biomaterials for scanning electron microscopy (SEM). Moreover, it is an adhesion promoter for photoresist in photolithography, and is also useful in the pyrolysis-gas chromatography-mass spectrometry to enhance the detectability of compounds with polar functional groups.??? ??
Hexamethyldisilazane, also known as HMDS, is an important organosilicon compound, a colorless and transparent liquid. It is readily hydrolyzed and gives off NH3 to produce hexamethyldisilyl ether. In the presence of a catalyst, it reacts with alcohols or phenols to produce trimethylalkoxysilane or trimethylaroxysilane. Reacts with anhydrous hydrogen chloride, releasing NH3 or NH4Cl, to produce trimethylchlorosilane.??? ??
Colorless transparent and easy flowing liquid. Boiling point 125℃, relative density 0.76 (20/4℃). Soluble in organic solvents, it will be rapidly hydrolyzed in contact with air to form trimethylsilanol and hexamethyldisilyl ether.??
Hexamethyldisilazane mainly used as methyl silane alkylation (such as amikacin, penicillin, cephalosporins and kinds of penicillin derivatives), hydroxyl protectants of antibiotics. It is used as surface treatment agent of diatomite, white carbon black, titanium and blond additives of photoresist in the semiconductor industry. It is used as treatment agent of tearing strength resistance. It is used as a solvent in organic synthesis and organometallic chemistry. It is used as an adhesion promoter for photoresist in photolithography. it is used for the preparation of trimethylsilyl ethers from hydroxy compounds. It is used as an alternative to critical point drying during sample preparation in electron microscopy. It is added to analyte to get silylated diagnostic products during pyrolysis in gas chromatography- mass spectrometry.??
ChEBI: Hexamethyldisilazane is an N-silyl compound obtained from ammonia by replacement of two of the hydrogens with trimethylsilyl groups. Hexamethyldisilazane is a derivatisation agent used in gas chromatography mass spectrometry applications. It has a role as a chromatographic reagent. It derives from a hydride of an ammonia.?? ??
Hexamethyldisilazane appears as a liquid. May be toxic by ingestion. Irritates skin and eyes. Vapors are heavier than air. May emit highly toxic nitrogen oxide fumes when heated to decomposition. Used to deactivate chromatography support materials and to promote adhesion of photoresists in the electronics industry.??? ?? ??
Highly flammable. Moisture sensitive.?? ???
Hexamethyldisilazane reacts with many carbonyl containing organic compounds to generate gaseous ammonia. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides.???
Flammable, moderate fire risk.????
Inhalation or contact with material may irritate or burn skin and eyes. Fire may produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution.Synthesis
A production method for hexamethyldisilazane comprises the following steps: trimethylsilyl chloride is added into a sealed reaction kettle with a bearing pressure higher than 0.5MPa, stirring is carried out, ammonia gas is introduced, the ammonia pressure in the reaction kettle is maintained at a value between 0.1MPa and 0.2MPa, the temperature is between 40 ℃ and 50 ℃, the reaction is carried out, and the ammonia gas quantity is gradually reduced till the stop; and then, the pressure is maintained at the unchanged value between 0.1MPa and 0.2MPa, the reaction is maintained for 0.5h to 2h, then, the temperature in the kettle is lowered to a value below 10 ℃, water with the temperature lower than 10 ℃ is added, the dissolution reaction is carried out to generate ammonia chloride, and upper layer materials are hexamethyldisilazane after the delamination.Purification Methods
A possible impurity is Me3SiCl. Wash it well with pet ether and fractionate it through a vacuum jacketed column packed with Helipac using a reflux ratio of 10:1. [Langer et al. J Org Chem 23 50 1958, Beilstein 4 IV 4014.]?? ??
Deng, Xiang. "Hexamethyldisilazane." Synlett 2011.06(2011):881-882. Chiavari, G, D. Fabbri, and S. Prati. "Gas chromatographic-mass spectrometric analysis of products arising from pyrolysis of amino acids in the presence of hexamethyldisilazane." Journal of Chromatography A922.1–2(2001):235-241.Nation, J. L. "A new method using hexamethyldisilazane for preparation of soft insect tissues for scanning electron microscopy." Stain Technology 58.6(1983):347-51.
http://www.sigmaaldrich.com/catalog/product/sial/52619? lang=en®ion=US
http://www.sigmaaldrich.com/catalog/product/aldrich/40215? lang=en®ion=US
http://www.sigmaaldrich.com/catalog/product/sial/86944? lang=en®ion=US
????????? ?? ?? ? ???
???
?? ??
O,O'-BIS(TRIMETHYLSILYL)-5-FLUOROURACIL
??-?????????(MAP)
6-???-1H-???[2,3-B]???
Lithium bis(trimethylsilyl)amide
???
(2R)-2-[(4-Ethyl-2,3-dioxopiperazinyl)carbonylamino]-2-phenylacetic acid
3-METHOXYBENZAMIDINE HYDROCHLORIDE
(2R)-2-[(4-Ethyl-2,3-dioxopiperazinyl)carbonylamino]-2-(4-hydroxyphenyl)acetic acid
6-CHLORO-1H-PYRROLO[2,3-B]PYRIDINE
Sodium bis(trimethylsilyl)amide
Imidodisulfurylfluoride
???????
Imidodisulfurylchloride
N-(Trimethylsilyl)-2-[(trimethylsilyl)oxy]pyrimidin-4-amine
N,N'-??(??????)??????
????????? ?? ??
???( 719)?? ??
| ??? | ?? | ??? | ?? | ?? ? | ?? |
|---|---|---|---|---|---|
| Ningxia Jinhua Chemical Co.,Ltd | 025-52279164 |
info@nxjhchem.com | China | 79 | 58 |
| Wuhan Kemi-Works Chemical Co., Ltd. | +86-2785736489 +8618171418573 |
sales@kemiworks.com | China | 729 | 58 |
| Zouping mingyuan import & export trading co., ltd | +86-0543-2240078 +8618364991597 |
myzhao793@163.com | China | 994 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 |
sales1@chuanghaibio.com | China | 5884 | 58 |
| Iota silicone oil (Anhui) Co.,ltd. | +86-15255260163 |
sales05@siliconeoil.net | China | 311 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 |
abby@weibangbio.com | China | 8803 | 58 |
| Hebei Mujin Biotechnology Co.,Ltd | +86 13288715578 +8613288715578 |
sales@hbmojin.com | China | 12825 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531153977 |
allison@yan-xi.com | China | 5855 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 |
deasea125996@gmail.com | China | 2472 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 |
sales@capot.com | China | 29792 | 60 |
????????? ?? ??:
???????????? N-??-2-????? ??????? ?????????? ?????? 1,3-??(??????)?? ?? ?? ? ?? ? ?? ?????? 3-(????????)????? 3- ??? ?? ??? ?? ? ?? ?? ??? ? ???? ??? ??? ?? ??? ???-1,1,3,3-????????? 1,3-???-1,1,3,3-????????? ?????????
Octamethylcyclotetrasilazane
llthium(hexamethyldisilazane),LITHIUM HEXAMETHYLDISILAZANE,HEXAMETHYLDISILAZANE LITHIUM SALT,1,1,1,3,3,3-HEXAMETHYLDISILAZANE LITHIUM SALT