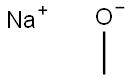??? ???
|
|
??? ??? ??
- ???
- -98 °C
- ?? ?
- 65 °C
- ??
- 0.97 g/mL at 20 °C
- ?? ??
- 500-600kg/m3
- ?? ??
- 1.1 (vs air)
- ???
- 50 mm Hg ( 20 °C)
- ???
- 1.3700
- ???
- 11 °C
- ?? ??
- Store at +5°C to +30°C.
- ???
- ???, ???, ?? ? ????? ?? ?????. ???? ? ?? ??? ???? ????.
- ??? ??
- ??
- ?? ?? (pKa)
- 15.17[at 20 ℃]
- ??
- ??
- Specific Gravity
- 0.945
- ??????(pH)
- 11 (20°C, 20g/L in H2O)
- ????
- 36%
- ???
- ?? ??
- ??
- Moisture Sensitive
- Hydrolytic Sensitivity
- 8: reacts rapidly with moisture, water, protic solvents
- Merck
- 14,8643
- BRN
- 3592982
- Dielectric constant
- 1.5(Ambient)
- ?? ??
- ACGIH: Ceiling 2 mg/m3
OSHA: TWA 2 mg/m3
NIOSH: IDLH 10 mg/m3; Ceiling 2 mg/m3
- ???
- ??? ???? ????. ?? ???? ???. ??? ??? ??? ??????. ?, ?, ??? ??? ???? ????.
- InChIKey
- WQDUMFSSJAZKTM-UHFFFAOYSA-N
- LogP
- -0.77 at 25℃
- CAS ??????
- 124-41-4(CAS DataBase Reference)
- ?? ? ?? ??
- ?? ? ???? ?? (GHS)
| ??? ?? | F,T,C | ||
|---|---|---|---|
| ?? ???? ?? | 11-23/24/25-34-39/23/24/25-36/38-14-36/37/38-22-10 | ||
| ????? | 8-16-26-43-45-7-36/37-7/8-36/37/39 | ||
| ????(UN No.) | UN 1431 4.2/PG 2 | ||
| WGK ?? | 1 | ||
| RTECS ?? | PC3570000 | ||
| F ?????? | 9-21 | ||
| ?? ?? ?? | 878 °F | ||
| TSCA | Yes | ||
| ?? ?? | 4.2 | ||
| ???? | II | ||
| HS ?? | 29051900 | ||
| ?? ?? ??? | 124-41-4(Hazardous Substances Data) | ||
| ?? | LD50 orally in Rabbit: 2037 mg/kg LD50 dermal Rat > 2000 mg/kg | ||
| ???? ?? | KE-23196 |
??? ??? C??? ??, ??, ??
????
? (1) ?? : ? ?? 5g? ?? ??? ?? ?? ??? ?? ?? 100mL? ? ?? ?????? ??. ???? 20mL? ?? ??? ?? ? 30mL? ?? ?, ? ?? ?? ?? ????? ??.
??(2) ?? : ? ??? ?????? ?? ??? ?, ? ?? 4.0ppm ????? ??.
??(3) ? : ? ?? 5.0g? ??? ??????? ?? ?????????????? ?? ??? ?, ? ?? 5.0ppm ????? ??.
??(4) ?? : ? ??? ?????? ?? ??? ?, ? ?? 1.0ppm ????? ??.
??(5) ????? : ??? (3)? ?? ????(Na2CO3?? 0.5% ??).
??(6) ?????? : ??? (4)? ?? ????(NaOH?? 2.0% ??).
????
? (1) ? ??? ???(1→100)? ??????.
??(2) ? ??? ???(1→100) 1??? ??(1→20) 0.1mL ? ???????? (1→300) 0.2mL? ??? 5?? ??? ?? ?? ??????????(1→4) 0.2mL ? ?? 3mL? ???? ?? ????????? 0.2mL? ?? ?, ?? ??~??? ????.
??(3) ? ??? ????? ? ????? ??? ????.
???
? (1) ???????????? ???? ? ?? 0.5g? ??? ???? ?? ?? ?????????? 10mL? ???? ??? ? ?? ??? ?? ? ?????(?—???)?? ????? ?? ????. ??, ?????????? 10mL? ???? ???? ??? ?? ???? ?? ?????? ? ???????? ?(A)? ??????(NaOH)??? ???.
a : ???? ??????? ???(mL)
b : ???? ??????? ???(mL)
f : ?????? 1mL? ???? ?? mg?
?????????? : ???? 10g? ????? ??? 100mL? ???. ?? ? ????.
??(2) ????????? ???? ? ?? 2g? ??? ???? ?? ??, ?? ?? ??? ?? ? 50mL? ??? ???. ? ?? ??????(3→25) 10mL? ???? ??? ? ?? 5?? ???? ? 1N ???? ????(??? : ???????? 2??) ?? ???? ?? ???????? ? ????????? ?(B)? ????????(CH3ONa)??? ???.
????????????????
??(3) (2)? ???? ?? 1N ?? 1mL? ???? ???? ? 5?? ???? ?? ?? ??? ?? 0.1N ?????????? ???? ?? ???? ?? ?????(Na2CO3)? ??(C)? ???.
??(4) ?? ???? ?? ??????(NaOH)? ??(D)? ???.
? ? ? ? D(%) = A—(C × 0.377)
??(5) ?? ???? ?? ????????(CH3ONa)? ??(E)? ???.
? ? ? ? E(%) = B—(D × 1.350)
??? ??
white powder??
Sodium methoxide is mainly used as a condensation agent, a strong alkaline catalyst and a methoxylating agent for the production of vitamin B1 and A, sulfadiazine and other drugs, and in small quantities for the production of pesticides. It is also used as a catalyst for treatment ofedible fats and oils, as an intermediate inmany synthetic reactions, to prepare sodiumcellulosate; and as a reagent in chemicalanalysis.?? ??
Sodium methylate is a white amorphous powder. It reacts with water to form sodium hydroxide, a corrosive material, and methyl alcohol, a flammable liquid. The heat from this reaction may be sufficient to ignite surrounding combustible material or the sodium methylate itself if the water is present in only small amounts. It is used to process edible fats and oils, and to make other chemicals.??? ?? ??
Highly flammable. Ignites in moist air [Wischmeyer 1966]. Reacts with water to produce a mixed solution of sodium hydroxide and methyl alcohol.?? ???
SODIUM METHYLATE is a strong base. Reacts with light metals forming H2 gas, with fire and explosion hazards. Too rapid addition of sodium methylate to a mixture of chloroform and methanol initiated an uncontrolled exothermic reaction between the chloroform and the methylate that caused a violent explosion [MCA Case History 693 1961]. Sodium methoxide is incompatible with 4-chloronitrobenzene and fluorinated cyclopropenyl methyl ethers, such as perfluoromethoxycyclopropene. The reactions are vigorous and may initiate ignition [Bretherick, 1995, pg. 191].???
(Solid) Flammable when exposed to heat or flame. (Solution) Flammable, moderate fire risk.????
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.????
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.Safety Profile
A corrosive and irritating material. It hydrolyzes into methanol and sodlum hydroxide. May ignite spontaneously in moist air. Flammable when exposed to heat or flame. Ignites on contact with water, Violent reaction with (CHCl3 + CH3OH), (methyl azide + dimethylmalonate), FClO3. When heated to decomposition it emits toxic fumes of Na2O.Purification Methods
It behaves in the same way as sodium ethoxide. It is hygroscopic and is hydrolysed by moist air to NaOH and MeOH. Material that has been kept under N2 should be used. If erratic results are obtained, even with recently purchased NaOMe, it should be freshly prepared thus: Clean Na (37g) cut in 1-3g pieces is added in small portions to stirred MeOH (800mL) in a 2L three-necked flask equipped with a stirrer and a condenser with a drying tube. After all the Na has dissolved, the MeOH is removed by distillation under vacuum, and the residual NaOMe is dried by heating at 150o under vacuum and kept under dry N2 [Burness Org Synth 39 51 1959]. [Beilstein 1 IV 1227.]??? ??? ?? ?? ? ???
???
?? ??
??? ??? ?? ??
| ??? | ?? | ??? | ?? | ?? ? | ?? |
|---|---|---|---|---|---|
| Tianjin Zhongxin Chemtech Co., Ltd. | 86-22-66880623 +8618622897568 |
sales@tjzxchem.com | China | 571 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 |
sales1@chuanghaibio.com | China | 5884 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 |
sales@tnjchem.com | China | 34563 | 58 |
| Jinan Chengyuan Chemical Co. , Ltd. | +86-0531-88274992 +8615552568189 |
2408152070@qq.com | China | 10 | 58 |
| LEAP CHEM CO., LTD. | +86-852-30606658 |
market18@leapchem.com | China | 24727 | 58 |
| Yujiang Chemical (Shandong) Co.,Ltd. | +86-17736087130 +86-18633844644 |
catherine@yjchem.com.cn | China | 994 | 58 |
| Wuxi High Mountain Hi-tech Development Co.,Ltd. | +86-86-0510-85881806 +8613357920996 |
wuxihighmountain@gmail.com | China | 87 | 58 |
| Jinan Finer Chemical Co., Ltd | +86-531-88989536 +86-15508631887 |
sales@finerchem.com | China | 2958 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 |
abby@weibangbio.com | China | 8803 | 58 |
| Hebei Mujin Biotechnology Co.,Ltd | +86 13288715578 +8613288715578 |
sales@hbmojin.com | China | 12825 | 58 |