????
|
|
???? ??
- ???
- 242-248 °C
- ??
- 15.5 º (c=4, 15N formic acid)
- ?? ?
- 436.08°C (rough estimate)
- ??
- 1.2051 (rough estimate)
- ???
- 14.5 ° (C=4, 15mol/L Formic Acid)
- ?? ??
- 2-8°C
- ???
- ?? ???(96%)? ?? ??? ?? ???, ??? ??????? ?? ?? ????.
- ??? ??
- ??
- ?? ?? (pKa)
- pKa 3.19±0.01 (H2O t=25.0 I=0.100(NaCl))(Approximate);7.87±0.02(H2O t=25.0 I=0.100(NaCl))(Approximate)
- ??
- ???
- ??
- ??? ??? ?
- ??????(pH)
- pH(8g/l, 25℃) : 4.5~6.0
- ???
- ???, ????????? ?????. ?? ???? ?? ???? ????.
- Merck
- 14,839
- BRN
- 2223850
- ???
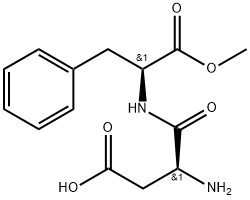
- H-Asp-Phe-OMe
- ???
- ????. ?? ???? ???? ????.
- InChIKey
- IAOZJIPTCAWIRG-QWRGUYRKSA-N
- LogP
- 0.542 (est)
- CAS ??????
- 22839-47-0(CAS DataBase Reference)
- ?? ? ?? ??
- ?? ? ???? ?? (GHS)
| ????? | 22-24/25 | ||
|---|---|---|---|
| WGK ?? | 2 | ||
| RTECS ?? | WM3407000 | ||
| TSCA | Yes | ||
| HS ?? | 29242990 | ||
| ?? ?? ??? | 22839-47-0(Hazardous Substances Data) | ||
| ?? | TDLo orl-wmn: 3710 mg/kg:SKN AIMEAS 104,207,86 |
???? C??? ??, ??, ??
??
????? ?? ???? ??? ????-?????-1-?? ???? ????, ?? ??? ??? ? 200?? ??? ??. ???? ????? ?? ???? ? ?? ?? ?? ??.??
??? ??? ? ??? ?? ??? ?? ?? ??? ??? 200?? 1 ??? ???? ?? ???, ?? ??? ?? ??? ???? ???? ??? ??. ??, ???? ??? ?? ??? ??? ?? ???.????
? (1) ?? : ? ?? 1g? 0.2N ???? ?? 100mL? ??? ?, ?? ????? ??.
??(2) ?? : ? ?? 0.8g? ?? ?? 100mL? ? ???? pH? 4.5~6.0??.
??(3) ???? : ? ?? 2g? ??? ?? 15N ?????? ?? ?? ??? 50mL? ? ?? 30???? ???? ???? ?? ???? ??? ?, 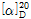 =+12.5~+17.5°??? ??.
=+12.5~+17.5°??? ??.
??(4) ?? : ? ??? ?????? ?? ??? ?, ? ?? 4.0ppm ????? ??.
??(5) ? : ? ?? 5.0g? ??? ??????? ?? ?????????????? ?? ??? ?, ? ?? 1.0ppm ????? ??.
??(6) 5-??-3,6-???-2-?????? : ? ?? 10mg? ??? ?? ?????? ?? ?? ???(? 3mL)? ??? ??????? 1mL? ??? ???? ??? ? 80℃?????? 30?? ??? ?? ???? ?? ? 15?? ??, ???? ????. ??, ???? 3mL? ?? ???? ??? ????? ?????? ?? ??????? 1mL? ??? ??? ?? ??? ???. ?? ?????????? ?? ??? ?, ? ?? 1.5% ????? ??.
?????
?????? ?? ? : ????? 220 ?? ?? ??? ?? ??? ?.
?????????? ? : ?? 3~4mm, ?? 2m? ???
????????? : 80~100??? ????? ?? ?? ??? ?????????? ??? ??? 3%?? ?? OV—1? ???.
?????? ?? ? : ?????? ???(FID)
????????? : 200℃
??? ???? : 200℃
????????? : 275℃
????????? ? ?? : ????? ????. 5-??-3,6-???-2-??????? 7~9??? ????? ??? ????.
??? ?
????????? : N,O-??(??????)??????, ???????? 3 : 2? ???? ????. ??? ????.
? ???? : 5-??-3,6-???-2-?????? ??? 25mg? ??? ?? 50mL ????? ??? ???? ?? 50mL? ??. ? ? 10mL? 100mL ????? ?? ???? 100mL? ???.
??(7) ??? : ? ??? 2N ???? 1% ??? ??? 1cm?? ?? ?????? 2N ??? ????? ?? 430nm?? ???? ??? ?, ? ???? 0.022 ????? ??.
????
? (1) ? ?? 10mg? ? 3mL ? ????????????(???? 2g? ??? ??????? 75mL? ??? ?? ?? ????? 62mg? ??? ??? 4M ????????(pH 9.0)? ??? 100mL? ? ?) 2mL? ??? ??? ?, ???? ????.
??(2) ? ?? ? 20mg? ??? 1mL? ??? ?????????? ???? ??? 0.5mL? ?? ?? ???? ??? 5N ?????????? 0.3mL? ???. ? ???? ?? ??? ???? ???? ?? 1N ???? pH? 1.0~1.5? ??? ?? ???????(1→100) 0.1mL? ?? ?, ???? ????.
???
? ? ??? ? 0.3g? ??? ?? ??? 3mL? ??? ??? ? ?? 50mL? ?? ?? ?? 0.1N ???????? ????(??? : α-???????? 0.5mL). ???? ?? ??? ???? ??? ??? ??. ?? ???? ????? ???? ?? ???? ????.
0.1N ?????? 1mL = 29.431mg C14H18N2O5
?????
? ? ??? ?????? 0.2% ????? ??.
??
Aspartame is the most popular artificial sweetener in the United States. It is sold as sweeteners such as NutraSweet and Equal, but it is also incorporated into thousands of food products.??? ??
Aspartame (N-L-aspartyl-L-phenylalanine-1-methyl ester, 3-amino-N-(a-carbomethoxy- phenethyl)-succinamic acid-N-methyl ester) is an intense sweetener widely used in foods and beverages. Its solubility in water is approximately 10 g/L at room temperature. Aspartame is not fully stable under common processing and storage conditions of foods and beverages with the highest stability around pH 4.3. Aspartame is about 200 times sweeter than sucrose with a clean, but slightly lingering sweetness. It is used as the single sweetener, but often also in blends with other intense sweeteners owing to synergistic taste enhancement and taste quality improvement often seen in such blends.In the European Union, aspartame is approved as E 951 for a large number of food applications. In the United States, it is approved as a multipurpose sweetener for food and beverage uses and it is also approved in many other countries.
??
Aspartame was discovered accidentally in 1965 during a search for drugs to treat gastric ulcers. James M. Schlatter, an organic chemist working for G. D. Searle & Company, was using aspartyl-phenylalanine methyl ester (aspartame) in a synthesis procedure and inadvertently got some of the compound on his hands.??
Aspartame is a high-intensity sweetener that is a dipeptide, provid- ing 4 cal/g. it is synthesized by combining the methyl ester of phenylalanine with aspartic acid, forming the compound n-l-alpha- aspartyl-l-phenylalanine-1-methyl ester. it is approximately 200 times as sweet as sucrose and tastes similar to sugar. it is compara- tively sweeter at low usage levels and at room temperature. its mini- mum solubility is at ph 5.2, its isoelectric point. its maximum solubility is at ph 2.2. it has a solubility of 1% in water at 25°c. the solubility increases with temperature. aspartame has a certain insta- bility in liquid systems which results in a decrease in sweetness. it decomposes to aspartylphenylalanine or to diketropiperazine (dkp) and neither of these forms is sweet. the stability of aspartame is a function of time, temperature, ph, and water activity. maximum stability is at approximately ph 4.3. it is not usually used in baked goods because it breaks down at the high baking temperatures. it contains phenylalanine, which restricts its use for those afflicted with phenylketonuria, the inability to metabolize phenylalanine. uses include cold breakfast cereals, desserts, topping mixes, chew- ing gum, beverages, and frozen desserts. the usage level ranges from 0.01 to 0.02%.??
ChEBI: A dipeptide composed of methyl L-phenylalaninate and L-aspartic acid joined by a peptide linkage.?? ??
Aspartame is produced by coupling together L-phenylalanine (or Lphenylalanine methyl ester) and L-aspartic acid, either chemically or enzymatically. The former procedure yields both the sweet aaspartame and nonsweet β-aspartame from which the α-aspartame has to be separated and purified. The enzymatic process yields only α-aspartame.?? ??
By coupling the amino acids L-phenylalanine and L-aspartic acid, and the esterification of the carboxyl group of the phenylalanine moiety to produce the methyl ester. This esterification can occur before or after coupling. The crystallized slurry is centrifuged and the resulting “wet-cake” is washed to remove impurities.?? ??
Asp-Phe methyl ester (aspartame, APM, ASP), a dipeptide ester, is made up of phenyl alanine and aspartic acid. Its genotoxic effects have been investigated. Its interaction with certain hydrocolloids has been studied.Pharmaceutical Applications
Aspartame is used as an intense sweetening agent in beverage products, food products, and table-top sweeteners, and in pharmaceutical preparations including tablets, powder mixes, and vitamin preparations. It enhances flavor systems and can be used to mask some unpleasant taste characteristics; the approximate sweetening power is 180–200 times that of sucrose.Unlike some other intense sweeteners, aspartame is metabolized in the body and consequently has some nutritive value: 1 g provides approximately 17 kJ (4 kcal). However, in practice, the small quantity of aspartame consumed provides a minimal nutritive effect.
Safety Profile
Human systemic effects byingestion: allergic dermatitis. Experimental reproductiveeffects. When heated to decomposition it emits toxicfumes of NOx.????
Aspartame is nontoxic. However, individuals with the rare, genetic disease, phenylketonuria (PKU), cannot properly metabolize phenylalanine. Such individuals are detected by testing at birth and placed on special low-phenylalanine diets to control their blood phenylalanine concentrations. Thus, PKU individuals need to be aware that aspartame is a source of phenylalanine.?? ?? ??
The rate of aspartame degradation is faster in a phosphate buffer solution than in a citrate buffer solution at the same pH and buffer concentration. The primary mechanism by which aspartame degrades, the formation of diketo piperazine, involves the nucleophilic attack of carbonyl by the free amine, which requires proton transfer.??
Aspartame is stable in dry conditions. In the presence of moisture, hydrolysis occurs to form the degradation products L -aspartyl-Lphenylalanine and 3-benzyl-6-carboxymethyl-2,5-diketopiperazine with a resulting loss of sweetness. A third-degradation product is also known, β-L-aspartyl-L-phenylalanine methyl ester. For the stability profile at 258℃ in aqueous buffers.Stability in aqueous solutions has been enhanced by the addition of cyclodextrins, and by the addition of polyethylene glycol 400 at pH 2. However, at pH 3.5–4.5 stability is not enhanced by the replacement of water with organic solvents.
Aspartame degradation also occurs during prolonged heat treatment; losses of aspartame may be minimized by using processes that employ high temperatures for a short time followed by rapid cooling.
The bulk material should be stored in a well-closed container, in a cool, dry place.
? ???
Differential scanning calorimetry experiments with some directly compressible tablet excipients suggests that aspartame is incompatible with dibasic calcium phosphate and also with the lubricant magnesium stearate. Reactions between aspartame and sugar alcohols are also known.Regulatory Status
Accepted for use as a food additive in Europe and in the USA. Included in the FDA Inactive Ingredients Database (oral powder for reconstitution, buccal patch, granules, syrups, and tablets). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.???? ?? ?? ? ???
???
?? ??
???? ?? ??
| ??? | ?? | ??? | ?? | ?? ? | ?? |
|---|---|---|---|---|---|
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618092446649 |
sarah@tnjone.com | China | 1143 | 58 |
| Shandong Deshang Chemical Co., Ltd. | +86-0531-8875-2665 +8613153039501 |
info@deshangchem.com | China | 662 | 58 |
| HebeiShuoshengImportandExportco.,Ltd | +86-18532138899 +86-18532138899 |
L18532138899@163.com | China | 939 | 58 |
| Aurora Industry Co., Ltd. | +86-13591747876 +86-13591747876 |
alex1_auco@126.com | China | 277 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 |
abby@weibangbio.com | China | 8810 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-81148696 +86-15536356810 |
1022@dideu.com | China | 3882 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 |
sales@hbmojin.com | China | 12835 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 |
sales1@chuanghaibio.com | China | 5889 | 58 |
| Sinoway Industrial co., ltd. | 0592-5800732; +8613806035118 |
xie@china-sinoway.com | China | 988 | 58 |
| Hebei Dangtong Import and export Co LTD | +86-13910575315 +86-13910575315 |
admin@hbdangtong.com | China | 1000 | 58 |