Propylamin Chemische Eigenschaften,Einsatz,Produktion Methoden
ERSCHEINUNGSBILD
FARBLOSE, HYGROSKOPISCHE FLüSSIGKEIT MIT CHARAKTERISTISCHEM GERUCH.
PHYSIKALISCHE GEFAHREN
Die D?mpfe sind schwerer als Luft und k?nnen sich am Boden ausbreiten. Fernzündung m?glich.
CHEMISCHE GEFAHREN
Zersetzung beim Verbrennen unter Bildung giftiger Rauche (Stickstoffoxide). Mittelstarke Base, reagiert mit S?uren und greift Aluminium und Zink an. Reagiert sehr heftig mit Oxidationsmitteln und Quecksilberunter Feuer- und Explosionsgefahr. Reagiert sehr heftig mit vielen Verbindungen wie halogenierte Kohlenwasserstoffe, Alkohole und einige Nitroparaffine. Greift Kupfer und Kupferlegierungen an.
ARBEITSPLATZGRENZWERTE
TLV nicht festgelegt (ACGIH 2005).
MAK nicht festgelegt (DFG 2005).
AUFNAHMEWEGE
Aufnahme in den K?rper durch Inhalation der D?mpfe und durch Verschlucken.
INHALATIONSGEFAHREN
Beim Verdampfen bei 20°C kann schnell eine gesundheitssch?dliche Kontamination der Luft eintreten.
WIRKUNGEN BEI KURZZEITEXPOSITION
WIRKUNGEN BEI KURZZEITEXPOSITION: Die Substanz ver?tzt die Augen, die Haut und die Atemwege.
LECKAGE
Gefahrenbereich verlassen! Fachmann zu Rate ziehen! Zündquellen entfernen. Ausgelaufene Flüssigkeit in abdichtbaren Beh?ltern sammeln. Verschüttete Flüssigkeit mit viel Wasser wegspülen. Pers?nliche Schutzausrüstung: Chemikalienschutzanzug mit umgebungsluftunabh?ngigem Atemschutzger?t.
R-S?tze Betriebsanweisung:
R11:Leichtentzündlich.
R20/21/22:Gesundheitssch?dlich beim Einatmen,Verschlucken und Berührung mit der Haut.
R34:Verursacht Ver?tzungen.
S-S?tze Betriebsanweisung:
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.
S36/37/39:Bei der Arbeit geeignete Schutzkleidung,Schutzhandschuhe und Schutzbrille/Gesichtsschutz tragen.
S45:Bei Unfall oder Unwohlsein sofort Arzt zuziehen (wenn m?glich, dieses Etikett vorzeigen).
S16:Von Zündquellen fernhalten - Nicht rauchen.
S7/9:Beh?lter dicht geschlossen an einem gut gelüfteten Ort aufbewahren.
S36:DE: Bei der Arbeit geeignete Schutzkleidung tragen.
Chemische Eigenschaften
n-Propylamine, also known as propylamine, is a colorless liquid with a pungent odor resembling ammonia. It has strong basic properties, leading to its easy formation of salts when combined with acids. Its reactivity is similar to that described for the other short chain aliphatic amines (Astel 1961).
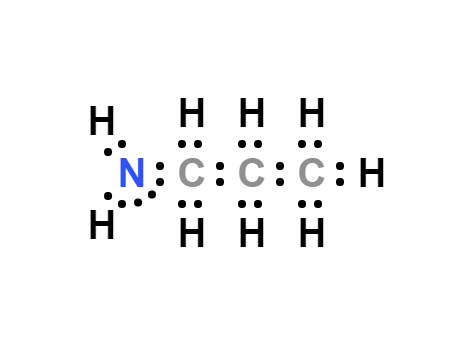
Verwenden
n-Propylamine is used as an intermediate inmany organic reactions. It has been used in the synthesis of single crystals of siliceous ferrierite.
Vorbereitung Methode
There are several methods employed in the manufacture of propylamine. Typically,
ammonia and propanol are reacted over a dehydration catalyst at high temperature
and pressure. Ammonia, propanol, and hydrogen can also be reacted over a
dehydrogenation catalyst such as metallic silver. Propylamine can also be synthesized
from propionaldehyde and ammonia with a Raney nickel catalyst (Schweizer
et al 1978).
Allgemeine Beschreibung
A clear colorless liquid with an ammonia-like odor. Flash point -35°F. Less dense than water and soluble in water. Vapors are heavier than air. Produces toxic oxides of nitrogen during combustion. Used in chemical analysis and to make other chemicals.
Air & Water Reaktionen
Highly flammable. Slightly soluble in water.
Reaktivit?t anzeigen
Colorless, alkaline liquid, very volatile (b. p. 48° C), moderately toxic, highly flammable. Dangerous fire hazard when exposed to heat, flame, sparks, or strong oxidizers. When heated to decomposition Propylamine emits toxic fumes of oxides of nitrogen. Incompatible with triethylaluminum, complex may explode on sublimation [Chini, P. et al., Chim. e Ind (Milan), 1962, 44, p. 1220].
Hazard
Highly flammable, dangerous fire risk,
explosive limits in air 2–10%, use alcohol foam
to extinguish. Strong irritant to skin and tissue.
Health Hazard
n-Propylamine is a strong irritant and a mod erately toxic substance. the toxic routes areinhalation, ingestion, and absorption throughthe skin. Contact of the liquid on the skincan cause burns and possibly skin sensitization. Irritation in the eyes from exposure tohigh concentrations can be severe. It is a respiratory tract irritant, similar to ethylamine.Inhalation of 2300 ppm of n-propylamine for4 hours caused labored breathing, hepatitis,and hepatocellular necrosis in rats. It is somewhat less toxic than ethylamine by oral anddermal routes.
LC50 value, inhalation (mice): 2500 mg/m
3/2 hr
LD50 value, oral (rats): 570 mg/kg
LD50 value, skin (rabbits): 560 mg/kg.
Brandgefahr
Highly flammable liquid; flash point (closed
cup) -37°C (-35°F); vapor density 2.0
(air = 1), vapor can travel a considerable distance to a source of ignition and flash back;
autoignition temperature 318°C (604°F); fire extinguishing agent: dry chemical, CO2, or
“alcohol” foam; water should be used to keep
fire-exposed containers cool and to flush and
dilute the spill.
n-Propylamine forms explosive mixtures
in air; LEL and UEL values are 2.0% and
10.4% by volume in air, respectively. There
is no report of explosion associated with this
compound. It is expected to exhibit violent
reactions characteristic of lower aliphatic
primary amines .
Industrielle Verwendung
Propylamine is important as a chemical intermediate for rubber chemicals, dyestuffs,
propyl isocyanate, textile and leather finishing resins and corrosion inhibitors.
It is also used in the production of pharmaceuticals such as Prilocaine,
pesticides including Profluralin and in petroleum additives. In 1976, 500 tons of
propylamine were manufactured in the U.S. (HSDB 1988).
Sicherheitsprofil
Moderately toxic by
inhalation, ingestion, and skin contact
routes. A skin and severe eye irritant.
Possibly a skin sensitizer. Very dangerous
fire hazard when exposed to heat, flame, or
oxidizers. Explosive in the form of vapor
when exposed to heat or flame. To fight
fire, use alcohol foam. When heated to
decomposition it emits toxic fumes of NOx.
Incompatible with triethynyl aluminum. See
also AMINES.
m?gliche Exposition
Propylamine is used to make textile
resins, drugs, pesticides, and other chemicals.
Stoffwechsel
To date, there are several studies which indicate that propylamine may be
metabolized in many animal species, including man. When propylamine hydrochloride
was administered to humans orally, little of the parent compound was
recovered in the urine (Rechenberger 1940). It has also been reported that dogs are
capable of metabolizing propylamine (Bernhard 1938). McEwen et al (1966)
demonstrated that monoamine oxidase, purified from rabbit serum, was capable of
oxidizing propylamine, although less actively than substituted phenylethylamines
such as dopamine. Further characterization revealed that the protonated amine is
bound by an unprotonated enzyme group, that the enzyme active site is composed
of hydrophobic residues, and that interaction of the amine residues determines
maximal velocity and affinity constant (McEwen and Sober 1967). Oxidation of
the amine could be stimulated by the presence of aliphatic alcohols which
apparently bond in a tertiary complex with the enzyme and substrate, thereby
increasing the effectiveness of substrate binding.
Other investigators have provided evidence that propylamine may not be a
substrate for tissue monoamine oxidase. When given intraperitoneally to rats it had
no effect on the liver enzyme and little effect on activity in the brain (Valiev 1974).
Early work by Pugh and Quastel (1937) indicated that slices of rat brain did not
metabolize propylamine.
Versand/Shipping
UN1277 Propylamine, Hazard Class: 3; Labels:
3-Flammable liquid, 8-Corrosive material.
l?uterung methode
Distil the amine from zinc dust, under reduced pressure, in an atmosphere of nitrogen. [Beilstein 4 IV 464.]
Inkompatibilit?ten
Propylamine Vapors may form explosive mixture with air. Violent reaction on contact with oxidizers and mercury, strong acids; organic anhydrides; isocyanates, aldehydes, nitroparrafins, halogenated hydrocarbons; alcohols and many other compounds. Attacks many metals and alloys, especially those of copper. Aqueous solution is acidic and may attack glass.
Waste disposal
Consult with environmental
regulatory agencies for guidance on acceptable disposal
practices. Generators of waste containing this contaminant
(≥100 kg/mo) must conform with EPA regulations
governing storage, transportation, treatment, and waste
disposal.
Propylamin Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

