Iodosylbenzol Chemische Eigenschaften,Einsatz,Produktion Methoden
S-S?tze Betriebsanweisung:
S17:Von brennbaren Stoffen fernhalten.
S36/37/39:Bei der Arbeit geeignete Schutzkleidung,Schutzhandschuhe und Schutzbrille/Gesichtsschutz tragen.
Chemische Eigenschaften
Iodosobenzene is an amorphous yellow substance; it explodes at 210℃, decomposing with the evolution of iodine vapour, and dissolves in hot water and alcohol. If acids do not oxidise C6H5IO, they give saline compounds in which iodosobenzene appears as a basic oxide of a diatomic metal, C6H5I. Thus, for instance, when an acetic acid solution of iodosobenzene is treated with a solution of nitric acid, it gives large monoclinic crystals of a nitric acid salt having the composition C6H5(NO3)2 [like Ca(NO3)2). Iodosobenzene displaces iodine from potassium iodide (in a solution acidulated with acetic or hydrochloric acid)-ie, it acts with its oxygen like HClO. The action of peroxide of hydrogen, chromic acid, and other similar oxidising agents gives C6H5IO2, which is a neutral substance-i.e, is incapable of giving salts with acids.
Iodosobenzene is one of the very first oxidants and remains in use because it has excellent oxygen-transfer behavior and mechanistic cleanliness (Hill & Schardt, 1980; Rezaeifard et al., 2007; Po?towicz et al., 2006).
The Principles of Chemistry Volume 1
Verwenden
Oxygen transfer reagent for stiochiometric or catalytic cross-functionalization of alkenes, alcohols, sulfides, and organometallo Compounds.
Iodosobenzene is used as an oxidizing and acetoxylating agent in organic synthesis. It is actively involved in the preparation of (Z)-3,7-dimethyl-2,6-octadien-1-al(neral) from (Z)-3,7-dimethyl-2,6-octadien-1-ol (nerol) in presence of 2,2,6,6-tetramethylpiperidin-1-oxyl (TEMPO). It is a useful reagent for the synthesis of a wide variety of heterocyclic compounds. It is also used in the Pd-catalyzed 2-arylation of indoles.
Synthese
Iodosobenzene has been prepared by the action of sodium or potassium hydroxide solution on iodobenzene dichloride and by addition of water to the dichloride.
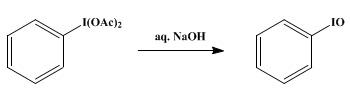
Iodosobenzene is prepared from iodobenzene.It is prepared by first oxidizing iodobenzene by peracetic acid. Hydrolysis of resulting diacetate affords "PhIO":
C6H5I + CH3CO3H + CH3CO2H → C6H5I(O2CCH3)2 + H2O
C6H5I(O2CCH3)2 + H2O → C6H5IO + 2CH3CO2H
http://orgsyn.org
Iodosylbenzol Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

