Trifluormethansulfonsaeureanhydrid Chemische Eigenschaften,Einsatz,Produktion Methoden
R-S?tze Betriebsanweisung:
R14:Reagiert heftig mit Wasser.
R21/22:Gesundheitssch?dlich bei Berührung mit der Haut und beim Verschlucken.
R34:Verursacht Ver?tzungen.
R35:Verursacht schwere Ver?tzungen.
S-S?tze Betriebsanweisung:
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.
S36/37/39:Bei der Arbeit geeignete Schutzkleidung,Schutzhandschuhe und Schutzbrille/Gesichtsschutz tragen.
S43:Zum L?schen . . . (vom Hersteller anzugeben) verwenden (wenn Wasser die Gefahr erh?ht, anfügen: "Kein Wasser verwenden").
S45:Bei Unfall oder Unwohlsein sofort Arzt zuziehen (wenn m?glich, dieses Etikett vorzeigen).
S8:Beh?lter trocken halten.
Beschreibung
Trifluoromethanesulfonic anhydride, also known as triflic anhydride, has proven to be an extraordinary reagent for a broad range of transformations. As a commercially and readily available reagent, It has been widely used in synthetic chemistry due to its high electrophilicity. Given its high affinity towards O-nucleophiles, reaction with alcohols, carbonyls, sulfur phosphorus- and iodine oxides towards the formation of the corresponding triflates is strongly favored. As one of the premier leaving groups in organic chemistry, the generated triflates then open the door to various downstream transformations, including (but not limited to) substitution reactions, cross-coupling processes, redox reactions, and rearrangements[1-2].
Chemische Eigenschaften
clear colorless to light brown liquid
Verwenden
Trifluoromethanesulfonic Anhydride is a strong electrophile used in chemical synthesis for introducing the triflyl group.
Hazard
May be corrosive to metals. Harmful if swallowed. Causes severe skin burns and eye damage.
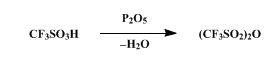
Synthese
The synthesis of Trifluoromethanesulfonic anhydride is as follows:
A dry, 100-ml., round-bottomed flask is charged with 36.3 g. (0.242
mole) of trifluoromethanesulfonic acid (Note 1) and 27.3 g. (0.192 mole)
of phosphorus pentoxide (Note 2). The flask is stoppered and allowed to
stand at room temperature for at least 3 hours. During this period the
reaction mixture changes from a slurry to a solid mass. The flask is
fitted with a short-path distilling head and heated first with a stream
of hot air from a heat gun and then with the flame from a small burner.
The flask is heated until no more trifluoromethanesulfonic anhydride
distills, b.p. 82–115°, yielding 28.4–31.2 g. (83–91%) of the anhydride,
a colorless liquid. Although this product is sufficiently pure for use
in the next step, the remaining acid may be removed from the anhydride
by the following procedure. A slurry of 3.2 g. of phosphorus pentoxide
in 31.2 g. of the crude anhydride is stirred at room temperature in a
stoppered flask for 18 hours. After the reaction flask has been fitted
with a short-path distilling head, it is heated with an oil bath,
yielding 0.7 g. of forerun, b.p. 74–81°, followed by 27.9 g. of the pure
trifluoromethanesulfonic acid anhydride, b.p. 81–84° .
Lager
Store in a cool, dry, wellventilated area. Moisture sensitive.
l?uterung methode
It can be freshly prepared from the anhydrous acid (11.5g) and P2O5 (11.5g, or half this weight) by setting aside at room temperature for 1hour, distilling off volatile products then distil it through a short Vigreux column. It is readily hydrolysed by H2O and decomposes appreciably after a few days to liberate SO2 and produce a viscous liquid. Store it dry at low temperatures. [Burdon et al. J Chem Soc 2574 1957, Beard et al. J Org Chem 38 373 1973, Beilstein 3 IV 35.]
Trifluormethansulfonsaeureanhydrid Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
5-AMINO-1,2,3-THIADIAZOLE-4-CARBOXYLIC ACID ETHYL ESTER
(S)-(-)-2,2'-BIS(DI-P-TOLYLPHOSPHINO)-1,1'-BINAPHTHYL
Troglitazone
1-FLUORO-4-(TRIFLUOROMETHYLTHIO)BENZENE
(S)-(-)-7,7'-BIS[DI(3,5-DIMETHYLPHENYL)PHOSPHINO]-2,2',3,3'-TETRAHYDRO-1,1'-SPIROBIINDANE
(S)-7,7'-Bis[di(p-methylphenyl)phosphino]-1,1'-spirobiindane ,97%
(R)-(+)-7,7'-BIS[DI(4-METHYLPHENYL)PHOSPHINO]-2,2',3,3'-TETRAHYDRO-1,1'-SPIROBIINDANE
(S)-(-)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl
3,5-DIMETHYLISOXAZOL-4-YL ISOCYANATE
(R)-(+)-7,7'-BIS(DIPHENYLPHOSPHINO)-2,2',3,3'-TETRAHYDRO-1,1'-SPIROBIINDANE
N-Phenyl-bis(trifluoromethanesulfonimide)
2-(TRIMETHYLSILYL)PHENYL TRIFLUOROMETHANESULFONATE
(R)-(+)-TolBINAP
1-(3-AMINO-PYRIDIN-2-YL)-ETHANONE
(R)-7,7'-BIS(DIPHENYLPHOSPHINO)-1,1'-SPIROBIINDANE

