1135-66-6
中文名稱
異長葉烯
英文名稱
Isolongifolene
CAS
1135-66-6
EINECS 編號
214-494-2
分子式
C15H24
MDL 編號
MFCD00042616
分子量
204.35
MOL 文件
1135-66-6.mol
更新日期
2025/03/26 09:00:25
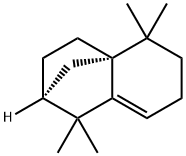 1135-66-6 結(jié)構(gòu)式
1135-66-6 結(jié)構(gòu)式
基本信息
中文別名
(2S)-1,3,4,5,6,7-六氫-1,1,5,5-四甲基-2H-2,4A-亞甲基萘異長葉烯
(-)-異長葉烯
英文別名
(1R)-2,2,7,7-TETRAMETHYLTRICYCLO[6.2.1.0(1.6)]UNDEC-5-ENE(-)-ISOLONGIFOLENE
ISOLONGIFOLENE
(-)-Isolongifoline
(2s)-1,3,4,5,6,7-hexahydro-1,1,5,5
(2s)-1,3,4,5,6,7-hexahydro-1,1,5,5-tetramethyl-2h-2,4a-methanonaphthalene
2H-2,4a-Methanonaphthalene, 1,3,4,5,6,7-hexahydro-1,1,5,5-tetramethyl-
2H-2,4a-Methanonaphthalene, 1,3,4,5,6,7-hexahydro-1,1,5,5-tetramethyl-, (2S,4aR)-(-)-
2H-2,4a-Methanonaphthalene, 1,3,4,5,6,7-hexahydro-1,1,5,5-tetramethyl-, (2S-cis)-
3,4,5,6,7-hexahydro-1,1,5,5-tetramethyl-4a-methanonaphthalen(2s)-2h-1
4a-Methanonaphthalene,1,3,4,5,6,7-hexahydro-1,1,5,5-tetramethyl-,(2S)-2H-2
Isolongipholene
C15 H24, 2H-2,4a-Methanonaphthalene, 1,3,4,5,6,7-hexahydro-1,1,5,5-tetramethyl-, (2S)-
ISOLONGIFOLENE(SG)
(2s)-2h-1,3,4,5,6,7-hexahydro-1,1,5,5-tetramethyl-4a-methanonaphthalen
1,3,4,5,6,7-Hexahydro-1,1,5,5-tetramethyl-2H-2,4a-methanonaphthalene
ISOLONGIFOLENE WITH GC
(2S,4aR)-1,2,3,4,4a,5,6,7-Octahydro-1,1,5,5-tetramethyl-2,4a-methanonaphthalene
(2S,4aR)-1,3,4,5,6,7-Hexahydro-1,1,5,5-tetramethyl-2H-2,4a-methanonaphthalene
(2S,4aR)-1,3,4,5,6,7-Hexahydro-1,1,5,5-tetramethyl-2H-2α,4aα-methanonaphthalene
所屬類別
香精與香料:萜烯烴物理化學(xué)性質(zhì)
外觀性質(zhì)微黃色液體,不溶于水,溶于乙醇等有機(jī)溶劑。沸點(diǎn):113~114℃折光率:1.499密度:0.930閃點(diǎn):49 °C
沸點(diǎn)255-256 °C
密度0.930 g/mL at 20 °C(lit.)
蒸氣壓5.49Pa at 25℃
折射率n20/D 1.499
閃點(diǎn)49°C
儲存條件2-8°C
溶解度DMSO: 10 mg/mL (48.94 mM)
形態(tài)Liquid
顏色Colorless to light yellow
氣味 (Odor)at 100.00 %. woody dusty amber incense
香型woody
旋光性 (optical activity)[α]20/D 138±2°, c = 1% in ethanol
水溶解性59.998μg/L at 25℃
BRN2207559
LogP5.77 at 25℃
CAS 數(shù)據(jù)庫1135-66-6(CAS DataBase Reference)
安全數(shù)據(jù)
警示詞警告
危險(xiǎn)性描述H225-H301+H311+H331-H370
危險(xiǎn)類別碼R10
危險(xiǎn)品運(yùn)輸編號UN 2319 3/PG 3
WGK Germany3
危險(xiǎn)等級3.2
包裝類別III
異長葉烯價(jià)格(試劑級)
| 報(bào)價(jià)日期 | 產(chǎn)品編號 | 產(chǎn)品名稱 | CAS號 | 包裝 | 價(jià)格 |
| 2025/02/08 | HY-N7363 | 異長葉烯 Isolongifolene | 1135-66-6 | 1 mg | 514元 |
| 2025/02/08 | HY-N7363 | 異長葉烯 Isolongifolene | 1135-66-6 | 5mg | 1350元 |
| 2025/02/08 | HY-N7363 | 異長葉烯 Isolongifolene | 1135-66-6 | 10 mM * 1 mLin DMSO | 1485元 |
常見問題列表
生物活性
Isolongifolene ((-)-Isolongifolene) 是一種從 Murraya koenigii 中分離的三環(huán)倍半萜烯。Isolongifolene 通過調(diào)節(jié) P13K/AKT/GSK-3β 信號通路來減輕魚藤酮誘導(dǎo)的氧化應(yīng)激,線粒體功能障礙和細(xì)胞凋亡。Isolongifolene 具有抗氧化,抗炎,抗癌和神經(jīng)保護(hù)的特性。體外研究
Isolongifolene (0-50 μM; 26 hours; SH-SY5Y neuroblastoma cells) treatment significantly alleviates Rotenone-induced cytotoxicity in SH-SY5Y cells in a dose-dependent manner.
Isolongifolene (10 μM; 26 hours; SH-SY5Y neuroblastoma cells) treatment attenuates Rotenone-induced apoptosis in SH-SY5Y cells.
Isolongifolene (10 μM; 26 hours; SH-SY5Y neuroblastoma cells) treatment attenuates Rotenone induced toxicity by down-regulating Bax, caspases-3, 6, 8 and 9 expression and up-regulating of Bcl-2 expression. Furthermore regulation of p-P13K, p-AKT and p-GSK-3β expression by Isolongifolene.
Cell Viability Assay
| Cell Line: | SH-SY5Y neuroblastoma cells |
| Concentration: | 0 μM, 1 μM, 2.5 μM, 5 μM, 10 μM, 20 μM and 50 μM |
| Incubation Time: | 26 hours |
| Result: | Significantly alleviated Rotenone-induced cytotoxicity in SH-SY5Y cells. |
Apoptosis Analysis
| Cell Line: | SH-SY5Y neuroblastoma cells |
| Concentration: | 10 μM |
| Incubation Time: | 26 hours |
| Result: | Attenuated Rotenone-induced apoptosis in SH-SY5Y cells. |
Western Blot Analysis
| Cell Line: | SH-SY5Y neuroblastoma cells |
| Concentration: | 10 μM |
| Incubation Time: | 26 hours |
| Result: | Attenuated rotenone induced toxicity by down-regulating Bax, caspases-3, 6, 8 and 9 expression and up-regulating of Bcl-2 expression. Prevented the rotenone-induced decreased phosphorylation of GSK-3β. |
