| Identification | More | [Name]
(+/-)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl | [CAS]
98327-87-8 | [Synonyms]
(+/-)-(1,1'-BINAPHTHALENE-2,2'-DIYL)BIS(DIPHENYLPHOSPHINE)
(+/-)-2,2'-BIS(DIPHENYLPHOSPHINO)-1,1'-BINAPHTHALENE
(+/-)-2,2'-BIS(DIPHENYLPHOSPHINO)-1,1'-BINAPHTHYL
2,2'-BIS(DIPHENYLPHOSPHINO)-1,1'-BINAPHTHYL
(+/-)-BINAP
BINAP
R(+)-(1,1'-BINAPHTHALENE-2,2'-DIYL)BIS(DIPHENYLPHOSPHINE)
R-(+)-1,1'-BINAPHTHYL-2,2'-DIPHENYL PHOSPHINE
R(+)-2,2'-BIS(DIPHENYLPHOSPHINO)-1,1'-BINAPHTHALENE
(R)-(+)-2,2'-BIS(DIPHENYLPHOSPHINO)-1,1'-BINAPHTHYL
(R)-(+)-2,2BIS(DIPHENYLPHOSPHINO)-1,1-BINAPHTHYL
(R)-2,2'-BIS(DIPHENYLPHOSPHINO)-1,1'-BINAPHTHYL
RAC-2,2'-BIS(DIPHENYLPHOSPHINO)-1,1'-BINAPHTHYL
RAC-2,2-BIS(DIPHENYLPHOSPHINO)-1,1-BINAPHTHYL
RAC-BINAP
RACEMIC-2,2'-BIS(DIPHENYLPHOSPHINO)-1,1'-BINAPHTHYL
RACEMIC-BINAP
(R)-(+)-BINAP
(R)-BINAP
S(-)-(1,1'-BINAPHTHALENE-2,2'-DIYL)BIS(DIPHENYLPHOSPHINE) | [EINECS(EC#)]
619-338-0 | [Molecular Formula]
C44H32P2 | [MDL Number]
MFCD00010805 | [Molecular Weight]
622.67 | [MOL File]
98327-87-8.mol |
| Chemical Properties | Back Directory | [Appearance]
White to light beige powder | [Melting point ]
283-286 °C(lit.)
| [Boiling point ]
724.3±55.0 °C(Predicted) | [storage temp. ]
Keep in dark place,Inert atmosphere,Room temperature | [solubility ]
Chloroform (Slightly, Sonicated), Dichloromethane (Slightly, Heated), | [form ]
Powder | [color ]
White to light beige | [Water Solubility ]
Soluble in tetrahydrofuran, benzene and dichloromethane. Slightly soluble in ether, methanol and ethanol. Insoluble in water. | [Sensitive ]
Air Sensitive | [Detection Methods]
GC | [Merck ]
14,1223 | [BRN ]
5321443 | [InChIKey]
MUALRAIOVNYAIW-UHFFFAOYSA-N | [CAS DataBase Reference]
98327-87-8(CAS DataBase Reference) | [Storage Precautions]
Air sensitive |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S22:Do not breathe dust .
S24/25:Avoid contact with skin and eyes .
S37/39:Wear suitable gloves and eye/face protection .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice . | [WGK Germany ]
3
| [F ]
8-10-23 | [TSCA ]
No | [HS Code ]
29319099 |
| Hazard Information | Back Directory | [Chemical Properties]
1.1'-Binaphthyl-2.2'-diphemyl phosphine is White to light beige powder
| [General Description]
Racemic version of BINAP. | [reaction suitability]
reaction type: Cross Couplings
reagent type: ligand
reaction type: Acylations
reagent type: ligand
reaction type: Arylations
reagent type: ligand
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reagent type: ligand
reaction type: C-C Bond Formation
reagent type: ligand
reaction type: Decarboxylations
reagent type: ligand
reaction type: Stille Coupling | [Purification Methods]
Dissolve the enantiomer in toluene, wash it with 30% aqueous NaOH, three times with H2O, dry (Na2SO4), evaporate to ~15% of its volume and add an equal volume of degassed MeOH. Collect the solid, wash it with MeOH and dry it at 80o/0.005mm for 6hours. Recrystallise it from a 1:1 mixture of toluene/EtOH to optical purity (m 241-242o) [Takaya et al. Org Synth 67 20 1989]. [Noyori & Takaya Acc Chem Res 2 3 345 1990, Kitamura et al. Org Synth 71 1 1993, Takaya et al. Org Synth 72 74 1995, Kitamura et al. J Org Chem 57 4053 1992.] |
| Questions and Answers (Q&A) | Back Directory | [Uses]
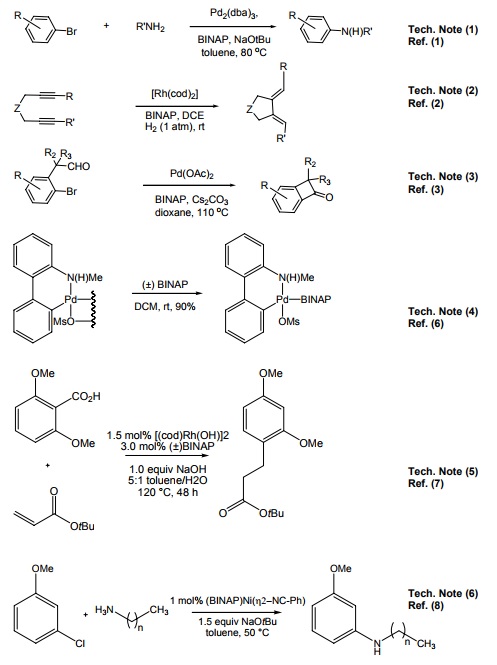
- racemic-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl ( (+/-)-BINAP ) is an essential role in the organic synthesis of enantioselective transformations catalyzed by the complexes of ruthenium, rhodium and palladium.
- It is also employed in palladium-catalyzed arylamine coupling in the preparation of demethylthiocholchines.
- used with Cu(II) to catalyze the addition of arylsulfonamides to styrenes and olefins.
| [Reactions]
Phosphine Ligand Kit component.
- Useful ligand for palladium-catalyzed carbon-nitrogen bond formation.
- Useful ligand for rhodium-catalyzed C-C bond formation.
- Useful ligand for palladium-catalyzed intramolecular acylation of aryl bromides via C-H activation.
- Used in the preparation of Buchwald third generation precatalyst.
- Used in methoxy directed Rhodium migration.
- Used in Nickel catalyzed C-N cross-coupling reactions.
|
|
|