| Identification | More | [Name]
Synephrine | [CAS]
94-07-5 | [Synonyms]
1-[4-HYDROXYPHENYL]-2-METHYLAMINOETHANOL
4-hydroxy-alpha-(methylaminomethyl)benzyl alcohol
AKOS NCG1-0008
AURORA KA-6561
DL-SYNEPHRINE
oxedrine
SYNEPHERINE
(+/-)-SYNEPHRINE
SYNEPHRINE
1-(4-hydroxyphenyl)-n-methylethanolamine
4-hydroxy-alpha-((methylamino)methyl)benzenemethanol
alpha-(4-oxyphenyl)alpha-oxy-beta-methylaminoaethan
analeptin
beta-methylamino-alpha-(4-hydroxyphenyl)ethylalcohol
ethaphene
methylaminomethyl(4-hydroxyphenyl)carbinol
parakorper
parasympatol
pentedrin
p-hydroxy-alpha-((methylamino)methyl)-benzylalcoho | [EINECS(EC#)]
202-300-9 | [Molecular Formula]
C9H13NO2 | [MDL Number]
MFCD00002370 | [Molecular Weight]
167.21 | [MOL File]
94-07-5.mol |
| Chemical Properties | Back Directory | [Appearance]
Off-White to Beige Powder | [Melting point ]
187 °C (dec.)(lit.)
| [Boiling point ]
295.79°C (rough estimate) | [density ]
1.1222 (rough estimate) | [refractive index ]
1.4680 (estimate) | [storage temp. ]
2-8°C
| [solubility ]
DMSO (Slightly), Methanol (Slightly, Sonicated) | [form ]
neat | [pka]
pKa 9.60±0.02(H2O
t = 25.0±0.5
I = 0.01)(Approximate) | [color ]
White to Off-White | [Usage]
A a-adrenergic receptor agonist, vasoconstrictor | [Merck ]
9012 | [Stability:]
Hygroscopic | [InChI]
InChI=1/C9H13NO2/c1-10-6-9(12)7-2-4-8(11)5-3-7/h2-5,9-12H,6H2,1H3/t9-/s3 | [InChIKey]
YRCWQPVGYLYSOX-DJEYLCQNNA-N | [SMILES]
C1(=CC=C(O)C=C1)[C@@H](O)CNC |&1:7,r| | [LogP]
-0.450 | [CAS DataBase Reference]
94-07-5(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing . | [WGK Germany ]
3
| [RTECS ]
DO7350000 | [HS Code ]
29225000 |
| Hazard Information | Back Directory | [Chemical Properties]
Off-White to Beige Powder | [Uses]
A a-adrenergic receptor agonist, vasoconstrictor | [Uses]
A α-adrenergic receptor agonist, vasoconstrictor. | [Uses]
anti-obesity | [Application]
Synephrine is a α-adrenergic receptor agonist, vasoconstrictor that has been reported to have thermogenic properties. Therefore, it is widely used as a dietary supplement to enhance energy and promote weight loss. It is reported that the (-)-form of synephrine has more potent than that of the (+)-form. (The product is for research purpose only.) | [Definition]
ChEBI: A phenethylamine alkaloid that is 4-(2-aminoethyl)phenol substituted by a hydroxy group at position 1 and a methyl group at the amino nitrogen. | [Synthesis Reference(s)]
Journal of the American Chemical Society, 71, p. 1045, 1949 DOI: 10.1021/ja01171a079 | [General Description]
Synephrine is a sympathomimetic amine from the plant?Citrus aurantium and is identified as a major active component of dietary supplements. | [Biochem/physiol Actions]
Synephrine is naturally found in citrus plants belonging to the Rutaceae family. It is a sympathomimetic alkaloid. Synephrine has 3 isomeric forms, such as m-synephrine, p-synephrine and o-synephrine. It exhibits β-adrenergic properties. | [storage]
Store at -20°C |
| Questions And Answer | Back Directory | [Plant Extract]
Synephrine is a kind of biological active substance, mainly extracted from the orange, tangerine peel, green husk and rutaceae plants, is a traditional Chinese medicine and plays an important role of frutus aurantii immaturus effective component, has a strong heart, increase blood volume contraction, blood vessels, high total peripheral vascular resistance and make the left ventricular pressure and the effect of arterial blood pressure, shock resistance, and the effect that reduce weight, but because the Synephrine has the similar structure to that of jute alkali has excited central nervous, the potential side effects of cardiovascular disease, in recent years it is limited to use in the United States, Canada and other countries.

Figure 1 the extract of frutus aurantii immaturus Synephrine | [Introduction]
Synephrine[meaning p-synephrine in most cases], is an alkaloid, occurring naturally in some plants and animals, and has been also supplemented in approved drugs products as its m-substituted analog known as neo-synephrine[1].
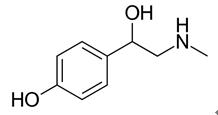
Figure 1 Chemical structure of the synephrine. ;
Synephrine[Figure 1] is a key proto-alkaloid found in Citrus aurantium[bitter orange] and other Citrus species[C. reticulata, C. sinensis, C. deliciosa, C. limon, C.limonia, and C. unshiu] as well as in Evodia rutaecarpa[2-5]. It is widely used in weight management and sports performance products. Moreover, it is usually consumed in various foods and juices derived from Citrus species as Seville oranges, mandarin oranges and many other orange-related species containing p-synephrine[7,8]. However, p-synephrine may cause potential cardiovascular hazards as found in many previous studies[9-11].
P-synephrine[P-s], often referred to as simply synephrine[12], is an alpha-adrenergic agonist[13] that also has some beta-adrenergic properties[14]. P-s occurs naturally in the human body in small quantities and may act as a neurotransmitter[15]. Since 1927, usually under the name oxedrine, it has been used as a pharmaceutical[16], commonly in the form of eye drops. Oxedrine is thought to be the primary ingredient in C. aurantium that produces weight loss, but neither this nor whether C. aurantium produces weight loss in humans is firmly established. M-synephrine, often referred to as phenylephrine, is an isomer of p-s. It is also an alpha -adrenergic agonist that also has some beta-adrenergic agonist properties. It has been studied far more extensively than p-s and is one of the two most widely used OTC decongestants today[12].
In Asian traditional medicine, synephrine was used in the treatment of digestive problems, to stimulate appetite and gastric secretion, in clear contrast with the aim of promoting weight-loss[17]. In the Mediterranean region, it was used as a cardiac and vascular stimulant, with stomachic, sedative, and general tonic properties[18]. Its most important and famous application is used for weight management, for the treatment of obesity. | [Applications]
The most famous application of synephrine is used for the treatment of obesity. Obesity is considered a growing public health issue: more than 1 billion adults are overweight and at least 300 million people are obese[BMIP30 kg/m2][19]. These situations lead to increased morbidity and mortality due to diseases such as diabetes mellitus, cardiovascular diseases, hypertension, and cancer[19, 20].
Due to its affinity to a-adrenergic receptors, p-synephrine is marketed as an intravenous drug to treat hypotension[17, 21]. m-Synephrine has been widely employed in parenteral formulations as an intranasal decongestant[22, 23], a vasopressor agent used in surgical procedures, in priapism treatment, and as a mydriatic agent[21].
| [Mode of action]
Both m- and p-synephrine activate several types of adrenergic receptors[21, 24]. Synephrine’s mechanism of action as a weight-loss stimulant is attributed to the lipolytic effects that occur with the activation of beta3-adrenergic receptors and consequent thermogenesis[Brunton et al., 2005; Moro and Basile, 2000]. Noteworthy, the occurrence of adverse effects associated with the consumption of synephrine is related to the nonspecific adrenergic stimulation[17].
The antidepressant effect of synephrine has already been described in rats through the tail suspension test and reserpine-induced hypothermia, two in vivo tests commonly used to screen for antidepressant activity[27]. Moreover, p-synephrine also demonstrated antidepressant effects through behavioral assays[tail suspension test and forced swimming test] in mice. The antidepressant-like activity in those assays was more pronounced at doses of 3 and 10 mg/kg[28]. A recent study demonstrated that synephrine acts on serotonergic receptors since its constrictive effects on rat aorta were concentration-dependently antagonized by pre-treatment with 5-HT1D and 5-HT2A antagonists[29].
| [Risks and Warning]
Since synephrine has structural and pharmacological similarities with other sympathomimetic amines and, therefore, it can cause similar side effects. Unfortunately, studies in the fields are still limited. Animal studies have shown that it can reduce the locomotor activity of mice[30,31]; reduce the relative mass of the adrenal glands in rats[2]. In human beings, there are reports that synephrine can cause ischemic colitis and seizure[21,31]. However, the biggest safety concern associated with synephrine is still related to its potential cardiovascular toxicity.
In Canada, between January 1998 and February of 2004, 16 cases of severe cardiovascular symptoms associated with weight-loss products containing synephrine were reported. These incidents determined synephrine’s interdiction in dietary supplements by Health Canada[32]. In 2010, Health Canada published new guidelines for the use of synephrine in natural products, which establish a daily limit of 30 mg as the maximum allowable dose for synephrine in these products[33]. The ergogenic mechanisms of sympathomimetic agents such as synephrine include increased heart rate and energy expenditure through central stimulation[34]. The users can undergo severe functional cardiac effects because of the increases in the after-load and heart rate, which can result in a higher myocardial oxygen demand, decreased diastolic filling, and failure to supply oxygen to the cardiomyocytes[36]. It has been also found that synephrine may cause a significant increase in systolic pressure[37] as well as promote an increase in total peripheral resistance during 30–60 min in healthy volunteers[35]. In vivo studies demonstrated an increase in mortality rate and electrocardiogram abnormalities in rats treated with synephrine for 15 days. The cardiac effects persisted for 15 subsequent days after the end of treatment[38]. These studies suggest a possible association between synephrine consumption and the occurrence of ventricular arrhythmias and increased cardiac output.
| [References]
- https://compendium.ch/mpro/mnr/22158/html/fr?Platform=Desktop&start=1
- Arbo, M.D., Franco, M.T., Larentis, E.R., Garcia, S.C., Sebben, V.C., Leal, M.B., Dallegrave, E., Limberger, R.P., 2008a. Arch. Toxicol., 95–99.
- M. D. Arbo, E. R. Larentis, V. M. Linck et al., “Concentrations of p-synephrine in fruits and leaves of Citrus species[Rutaceae] and the acute toxicity testing of Citrus aurantium extract and p-synephrine,” Food and Chemical Toxicology, vol. 46, no. 8, pp. 2770–2775, 2008.
- Avula, B., Upparapalli, S.K., Navarrete, A., Khan, I.A., 2005. Simultaneous quantification of adrenergic amines and flavonoids in Citrus aurantium, various Citrus species, and dietary supplements by liquid chromatography. J. AOAC Int. 88, 1593–1606.
- Chen, G., Zhang, L., Zhao, J., Ye, J., 2002. Determination of hesperidin and synephrine in Pericarpium Citri Reticulatae by capillary electrophoresis with electrochemical detection. Anal. Bioanal. Chem. 373, 169–173.
- Hibino, T., Yuzurihara, M., Kanno, H., Kase, Y., Takeda, A., 2009a. Goshuyuto, a traditional Japanese medicine, and aqueous extracts of Evodiae fructus constrict isolated rat aorta via adrenergic and/or serotonergic receptors. Biol. Pharm. Bull. 32, 237–241.
- B. C. Nelson, K. Putzbach, K. E. Sharpless, and L. C. Sander, “Mass spectrometric determination of the predominant adrenergic protoalkaloids in bitter orange[Citrus aurantium],” Journal of Agricultural and Food Chemistry, vol. 55, no. 24, pp. 9769–9775, 2007.
- L. C. Sander, K. Putzbach, B. C. Nelson et al., “Certification of standard reference materials containing bitter orange,” Analytical and Bioanalytical Chemistry, vol. 391, no. 6, pp. 2023–2034, 2008.
- S. Bent, A. Padula, and J. Neuhaus, “Safety and efficacy of citrus aurantium for weight loss,” American Journal of Cardiology, vol. 94, no. 10, pp. 1359–1361, 2004.
- S. R. Penzak, M. W. Jann, J. A. Cold, Y. Y. Hori, H. D. Desai, and B. J. Gurley, “Seville[sour] orange juice: synephrine content and cardiovascular effects in normotensive adults,” Journal of Clinical Pharmacology, vol. 41, no. 10, pp. 1059– 1063, 2001.
- A. Fugh-Berman and A. Myers, “Citrus aurantium, an ingredient of dietary supplementsmarketed for weight loss: current status of clinical and basic research,” Experimental Biology and Medicine, vol. 229, no. 8, pp. 698–704, 2004.
- National Toxicology Program. NTP toxicology and carcinogenesis studies of phenylephrine hydrochloride[CAS, No. 61-76-7] in F344/N rats and B6C3F1 mice[Feed Studies]. Natl Toxicol Program Tech Rep Series 1987; 322: 1–172.
- Brown CM, McGrath JC, Midgley JM, Muir AG, O’Brien JW, Thonoor CM, Williams CM, Wilson VG. Activities of octopamine and synephrine stereoisomers on alpha-adrenoceptors. Br J Pharmacol 1988; 93: 417–429.
- Jordan R, Midgley JM, Thonoor CM, Williams CM. Betaadrenergic activities of octopamine and synephrine stereoisomers on guinea-pig atria and trachea. J Pharm Pharmacol 1987; 39: 752–754.
- Kim KW, Kim HD, Jung JS, Woo RS, Kim HS, Suh HW, Kim YH, Song DK. Characterization of antidepressant-like effects of p-synephrine stereoisomers. Naunyn Schmiedebergs Arch Pharmacol 2001; 364: 21–26.
- Starke K. A history of Naunyn-Schmiedeberg’s archives of pharmacology. Naunyn Schmiedebergs Arch Pharmacol 1998; 358: 1–109.
- Fugh-Berman, A., Myers, A., 2004. Citrus aurantium, an ingredient of dietary supplements marketed for weight loss: current status of clinical and basic research. Exp. Biol. Med. 229, 698–704.
- Arias, B.A., Ramón-Laca, L., 2005. Pharmacological properties of citrus and their ancient and medieval uses in the Mediterranean region. J. Ethnopharmacol. 97, 89–95.
- WHO, 2009. World and Health Organization, Obesity and overweight.
- Allison, D.B., Fontaine, K.R., Manson, J.E., Stevens, J., Vanltallie, T.B., 1999. Annual deaths attributable to obesity in the United States. JAMA 282, 1530–1538.
- Haaz, S., Fontaine, K.R., Cutter, G., Limdi, N., Perumean-Chaney, S., Allison, D.B., 2006. Citrus aurantium and synephrine alkaloids in the treatment of overweigth and obesity: an update. Obes. Rev. 7, 79–88.
- Martindale, 2004. Phenylephrine, The Complete Drug Reference. Electronic version.London, UK.
- Santana, J., Sharpless, K.E., Nelson, B.C., 2008. Determination of p-synephrine and msynephrine positional isomers in bitter orange-containing supplements by LC/UV and LC/MS/MS. Food Chem. 109, 675–682.
- Torp, K.D., Tschakovsky, M.E., Halliwill, J.R., Minson, C.T., Joyner, M.J., 2001. B-Receptor agonist activity of phenylephrine in the human forearm. J. Appl. Physiol. 90, 1855–1859.
- Brunton, L., Lazo, J., Parker, K., 2005. In: Goodman, Gilman.[Ed.], ThePharmacological Basis of Therapeutics, 11th ed. McGraw-Hill.
- Moro, C.O., Basile, G., 2000. Obesity and medicinal plants. Fitoterapia 71, S73–S82.
- Kim, K.W., Kim, H.D., Jung, J.S., Woo, R.S., Kim, H.S., Suh, H.W., Kim, Y.L., Song, D.K., 2001. Characterization of antidepressant-like effects of p-synephrine stereoisomers. Naunyn Schmiedebergs Arch. Pharmacol. 364, 21–26.
- Song, D.K., Suh, H.W., Jung, J.S., Wie, M.B., Son, K.H., Kim, Y.H., 1996. Antidepressantlike effects of p-synephrine in mouse models of immobility tests. Neurosci. Lett. 214, 107–110.
- Hibino, T., Yuzurihara, M., Kase, Y., Takeda, A., 2009. Synephrine, a component of Evodiae fructus, constricts isolated rat aorta via adrenergic and serotonergic receptors. J. Pharmacol. Sci. 111, 73–81.
- Arbo, M.D., Larentis, E.R., Linck, V.M., Aboy, A.L., Pimentel, A.L., Henriques, A.T., Dallegrave, E., Garcia, S.C., Leal, M.B., Limberger, R.P., 2008b. Concentrations of p-synephrine in fruits and leaves of Citrus species[Rutaceae] and the acute toxicity testing of Citrus aurantium extract and p-synephrine. Food Chem. Toxicol. 46, 2770–2775.
- Sultan, S., Spector, J., Mitchell, R.M., 2006. Ischemic colitis associated with use of a bitter orange containing dietary weight-loss supplement. Mayo Clin. Proc. 81, 1630–1631.
- Jordan, S., Murty, M., Pilon, K., 2004. Products containing bitter orange or synephrine: suspected cardiovascular adverse reactions. Can. Med. Assoc. J. 171, 993–994.
- Health-Canada, 2010. Guidelines for the use of synephrine in natural health products. Available: <http://www.hc-sc.gc.ca>.
- Haller, C.A., Duan, M., Jacob 3rd, P., Benowitz, N., 2008. Human pharmacology of a performance-enhancing dietary supplement under resting and exercise conditions. Br. J. Clin. Pharmacol. 65, 833–840
- Thomas, J.E., Munir, J.A., McIntyre, P.Z., Ferguson, M.A., 2009. STEMI in a 24-yearsold man after use of a synephrine-containing dietary supplement: a case report and review of the literature. Text Heart Inst. J. 36, 586–590.
- Thomas, S.H., Clark, K.L., Allen, R., Smith, S.E., 1991. A comparison of the cardiovascular effects of phenylpropanolamine and phenylephrine containing proprietary cold remedies. Br. J. Clin. Pharmacol. 32, 705–711.
- Bui, L.T., Nguyen, D.T., Ambrose, P.J., 2006. Blood pressure and heart rate effects following a single dose of bitter orange. Ann. Pharmacother. 40, 53–57.
- Calapai, G., Firenzuoli, F., Saitta, A., Squadrito, F., Arlotta, M.R., Constantino, G., Inferrera, G., 1999. Antiobesity and cardiovascular toxic effects of Citrus aurantium extracts in the rat: a preliminary report. Fitoterapia 70, 586–592.
|
|
|