| Identification | Back Directory | [Name]
CHLORO(1,5-CYCLOOCTADIENE)(PENTAMETHYLCYCLOPENTADIENYL)RUTHENIUM (II) | [CAS]
92390-26-6 | [Synonyms]
Cp*RuCl(cod)
1,5-Cyclooctadiene, ruthenium complex
CHLORO(PENTAMETHYLCYCLOPENTADIENYL)(CYC&
Chloro(1��,5-cyclooctadiene)(pentamethylcyclopent...
Chloro(pentaMethylcyclopentadienyl)(cyclooctadiene)rutheniuM
Chloro(pentamethylcyclopentadienyl)(cyclooctadiene)ruthenium(II)
Chloro(1,5-cyclooctadiene)(pentaMethylcyclopentadienyl)rutheniuM
Chloro(1,5-cyclooctadiene)(η5-pentamethylcyclopentadienyl)ruthenium
CHLORO(1,5-CYCLOOCTADIENE)(PENTAMETHYLCYCLOPENTADIENYL)RUTHENIUM (II)
Chloro(1,5-cyclooctadiene)(pentamethylcyclopentadienyl)ruthenium(II),98%
Ruthenium,chloro[(1,2,5,6-h)-1,5-cyclooctadiene][(1,2,3,4,5-h)-1,2,3,4,5-pentamethyl-2,4-cyclopentadien-1-yl]- | [Molecular Formula]
C18H27ClRu 5* | [MDL Number]
MFCD07369035 | [MOL File]
92390-26-6.mol | [Molecular Weight]
379.93 |
| Questions And Answer | Back Directory | [Reactions]
- Catalyst for regio and stereo-specific ring opening via N-O bond cleavage.
- Catalyst for transformation of 1,6-enynes and diazoalkanes into alkenylbicyclo[3.1.0]hexane derivatives.
- Catalyst for ring closing enyne metathesis.
- Catalyst for [2 + 2 + 2] cocyclization of diene-yne, and cyclodimerization of allenynes.
- Catalyst for hydrovinylation of ynamides with ethylene.
- Catalyst for C–H insertion reactions of carbenes.
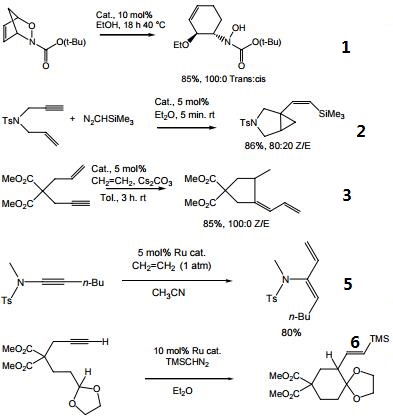
|
| Hazard Information | Back Directory | [Uses]
Chloro(pentamethylcyclopentadienyl)(cyclooctadiene)ruthenium(II) is a homogeneous catalyst for the formation of carbon-carbon and carbon-heteroatom bonds.
It can be used:
- To catalyze cyclotrimerization of alkynylboronates, propargyl alcohols, and terminal alkynes to form arylboronate, which in turn undergoes palladium(II)-catalyzed carbonylation to form highly substituted phthalides.
- To catalyze C-C coupling of norbornenes and norbornadiene with alkynes?to form [2 + 2] cycloadducts.
- In combination with 2-diphenylphosphinoethylamine-potassium tertiary butoxide to form a ternary catalyst system that can catalyze fast racemization of chiral non-racemic sec-alcohols.
- To synthesize new organoruthenium complexes with phosphorus-based ligands such as bis(phosphino)amines.
- To catalyze the addition of organic disulfides to alkenes leading to vic-dithioethers.
|
|
|