| Identification | Back Directory | [Name]
Chloro(1,5-cyclooctadiene)rhodium(I) dimer | [CAS]
12092-47-6 | [Synonyms]
[Rh(C8H12)Cl]2
bis(chlororhodium)
Di(chloro)bis(η
Chloro(1,5-cycloocta
bis((1z,5z)-cycloocta-1,5-diene)
4-cycloocta-1,5-diene)dirhodium(I)
Chloro(1,5-cyclooctadiene)rhodiuM(I)
Bis(1,5-cyclooctadienerhodium chloride)
Chloro(1,5-cyclooctadiene)rhodium dimer
CHLORO(1,5-CYCLOOCTADIENE)RHODIUM(I) DI&
(1,5-Cyclooctadiene)rhodiumchloridedimer
Dichloro-bis-1,5-cyclooctadiene dirhodium
Rhodium 1,5-cyclooctadiene chloride dimer
Rhodium chloro(cycloocta-1,5-diene) dimer
Bis(cycloocta-1,5-diene)dichlorodirhodium
CHLORO(1,5-CYCLOOCTADIENE)RHODIUM(I) DIMER
Chloro(1,5-cyclooctadiene)rhodium(Ⅰ) dimer
CHLORO(1,5-CYCLOOCTADIENE)RHODATE (I) DIMER
1,5-CYCLOOCTADIENE RHODIUM(I) CHLORIDE DIMER
Di-mu-chlorobis(1,5-cyclooctadiene)dirhodium
DICHLORO(1,5-CYCLOOCTADIENE)RHODIUM(I) DIMER
Chloro(1,5-cyclooctadiene)rhodium(I)dimer,98%
BIS(1,5-CYCLOOCTADIENE)DIRHODIUM(I) DICHLORIDE
Chloro(1,5-cyclooctadiene)rhodiuM(I) diMer 98%
Dichlorobis(1,5-cyclooctadiene)rhodiuM(I) diMer
DI-CHLORO-BIS-(CYCLOOCTA-1,5-DIENE)DIRHODIUM(I)
Chloro(1,5-cyclooctadiene)rhodium(I) dimmer,98%
Rhodium, di-mu-chlorobis(1,5-cyclooctadiene)di-
Bis(1,5-cyclohexadiene)-mu,mu'-dichlorodirhodium
BIS(1,5-CYCLOOCTADIENE)-MU,MU'-DICHLORO DIRHODIUM
Chloro(1,5-cyclooctadiene)rhodium(I), dimer, 99.9%
RHODIUM(I) CHLORIDE 1,5-CYCLOOCTADIENE COMPLEX DIMER
DI-MU-CHLOROBIS[(ETA-CYCLOOCTA-1,5-DIENE)RHODIUM (I)]
di-mu-chloro-bis(hapto-1,5-cyclooctadiene)dirhodium(I)
Di-μ-chlorobis[(1,2,5,6-η)-1,5-cyclooctadiene]dirhodium
Chloro(1,5-cyclooctadiene)rhodiuM(I) diMer,[Rh(COD)Cl]2
DI-MU-CHLOROBIS[(1,2,5,6-N)-1,5-CYCLOOCTADIENE]DIRHODIUM
Chloro(1,5-cyclooctadiene)rhodium(I) dimer, min. 40.8% Rh
Chloro-(15-cyclooctadiene)-rhodiuM(I)-diMer ([Rh(COD)CI]2
RhodiuM, di-M-chlorobis[(1,2,5,6-h)-1,5-cyclooctadiene]di-
RhodiuM, di-μ-chlorobis[(1,2,5,6-η)-1,5-cyclooctadiene]di-
di-mu-chlorobis[(1,2,5,6-eta)-1,5-cyclooctadiene]di-rhodiu
DI-MU-CHLORO-BIS[(1,2,5,6-ETA)-1,5-CYCLOOCTADIENE]DIRHODIUM
Chloro(1,5-cyclooctadiene)rhodiuM(I) diMer,98% [Rh(COD)Cl]2
Rhodium, di-mu-chlorobis[(1,2,5,6-eta)-1,5-cyclooctadiene]di-
Chloro(1,5-cyclooctadiene)rhodiuM(I) diMer, Min. 40.8% Rh 500MG
Chloro(1,5-cyclooctadiene)rhodiuM (I) diMer ,98% [C% : 38.62%-39.22%]
Chloro(1,5-cyclooctadiene)rhodiuM(I) diMer, 41% Rh, Product of UMicore
Chloro(1,5-cyclooctadiene)rhodiuM(Ⅰ) diMer, 41% Rh, Product of UMicore
Chloro(1,5-cyclooctadiene)rhodiuM(I) diMer/ Bis(1,5-cyclooctadienerhodiuM chloride)
Bis(1,5-cyclooctadiene)dirhodium(I) Dichloride
1,5-Cyclooctadiene Rhodium(I) Chloride Dimer
di-mu-chlorobis[(1,2,5,6-eta)-1,5-cyclooctadiene]di-rhodiu chloro(1,5-cyclooctadiene)rhodium(i) dimer
1,5-Cyclooctadienerhodium(I) chloride dimer, Chloro(1,5-cyclooctadiene)rhodium(I) dimer, Rhodium(I) chloride 1,5-Cyclooctadiene complex dimer
1,5-Cyclooctadienerhodium(I) chloride dimer, Bis(1,5-cyclooctadiene)dirhodium(I) dichloride, Rhodium(I) chloride 1,5-Cyclooctadiene complex dimer, Di-μ-chlorobis[(1,2,5,6-η:)-1,5-cyclooctadiene]dirhodium | [EINECS(EC#)]
235-157-6 | [Molecular Formula]
C16H24Cl2Rh2 | [MDL Number]
MFCD00012415 | [MOL File]
12092-47-6.mol | [Molecular Weight]
493.08 |
| Hazard Information | Back Directory | [Chemical Properties]
orange crystals | [Uses]
It is a widely used precursor to homogeneous catalysts. This is a chiral catalyst capable of asymmetrically hydrogenating certain prochiral alkenes. Chloro(1,5-cyclooctadiene)rhodium(I) dimer is also used in the synthesis of other metal ligands for use in catalysis. | [General Description]
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product has been enhanced for catalytic efficiency. Find details here. | [Flammability and Explosibility]
Notclassified | [reaction suitability]
core: rhodium
reagent type: catalyst |
| Questions And Answer | Back Directory | [Reaction]
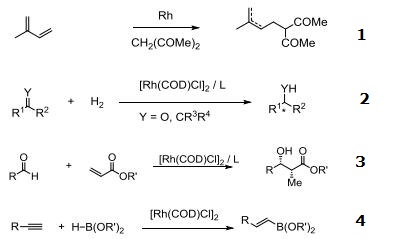
- Catalyst for coupling 1,3-dienes with activate methylene compounds.
- Rhodium source for various asymmetric hydrogenation systems and asymmetric hydrosilylation of ketones.
- Rhodium source for asymmetric reductive aldol reaction.
- Cis-hydroboration of terminal alkynes.
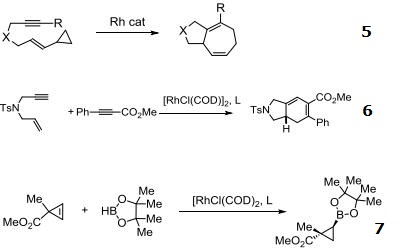
- Rhodium source for [5 + 2] additions.
- Highly enantioselective for [2+2+2] carbocyclization reactions.
- Enantioselective hydroboration of cyclopropenes.
|
|
|