| Identification | More | [Name]
Potassium tert-butanolate | [CAS]
865-47-4 | [Synonyms]
KTB
POTASSIUM T-BUTOXIDE
Potassium tert-butanolate
POTASSIUM TERT-BUTOXIDE
POTASSIUM TERT-BUTYLATE
2-methyl-2-propanopotassiumsalt
2-Propanol,2-methyl-,potassiumsalt
potassium-butoxide
potassiumt-butoxide,ktb
potassiumt-butoxide,ktb,in
potassiumt-butoxide,ktb,inisobutanol
potassiumt-butoxide,ktb,inisopropanol
potassiumt-butoxide,ktb,int-butanol
potassiumt-butoxide,ktb,intetrahydrofuran
Tert-butylalcohol,potassiumderivative
Potassium Tertiary Butoxide
Potassium tert-butoxide, in isobutanol
Potassium tert-butoxide, in isopropanol
Potassium tert-butoxide, in t-butanol
Potassium tert-butoxide, in tetrahydrofuran | [EINECS(EC#)]
212-740-3 | [Molecular Formula]
C4H9KO | [MDL Number]
MFCD00012162 | [Molecular Weight]
112.21 | [MOL File]
865-47-4.mol |
| Chemical Properties | Back Directory | [Appearance]
white crystalline powder | [Melting point ]
256-258 °C (dec.) (lit.) | [Boiling point ]
275°C | [density ]
0.910 g/mL at 20 °C
| [vapor pressure ]
1 mm Hg ( 220 °C)
| [Fp ]
54 °F
| [storage temp. ]
Flammables area | [solubility ]
Soluble in hexane, toluene, diethyl ether and terahydrofuran. | [form ]
Solution | [pka]
pK1:18(25°C) | [color ]
White to off-white | [Specific Gravity]
0.902 | [PH]
13 (5g/l, H2O, 20℃)Hydrolysis | [Stability:]
Stable, but reacts violently with water and acids, possibly leading to fire. Incompatible with water, acids, halogenated hydrocarbons, alcohols, strong oxidizing agents, ketones, carbon dioxide. | [Water Solubility ]
REACTS | [Hydrolytic Sensitivity]
8: reacts rapidly with moisture, water, protic solvents | [Sensitive ]
Moisture Sensitive | [Detection Methods]
T | [BRN ]
3556712 | [InChIKey]
LPNYRYFBWFDTMA-UHFFFAOYSA-N | [CAS DataBase Reference]
865-47-4(CAS DataBase Reference) | [Storage Precautions]
Moisture sensitive;Store under nitrogen | [EPA Substance Registry System]
865-47-4(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
F,C,Xi | [Risk Statements ]
R11:Highly Flammable.
R19:May form explosive peroxides.
R22:Harmful if swallowed.
R34:Causes burns.
R35:Causes severe burns.
R14:Reacts violently with water.
R20:Harmful by inhalation.
R36/37:Irritating to eyes and respiratory system . | [Safety Statements ]
S16:Keep away from sources of ignition-No smoking .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S43:In case of fire, use ... (indicate in the space the precise type of fire-fighting equipment. If water increases the risk add-Never use water) .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S7/9:Keep container tightly closed and in a well-ventilated place .
S8:Keep container dry .
S33:Take precautionary measures against static discharges .
S27:Take off immediately all contaminated clothing .
S29:Do not empty into drains . | [RIDADR ]
UN 3274 3/PG 2
| [WGK Germany ]
1
| [F ]
10-21 | [Autoignition Temperature]
483 °C | [Hazard Note ]
Flammable solid/Corrosive | [TSCA ]
Yes | [HazardClass ]
8 | [PackingGroup ]
I | [HS Code ]
29051900 |
| Raw materials And Preparation Products | Back Directory | [Raw materials]
tert-Butanol | [Preparation Products]
4-PYRIDIN-2-YLISOXAZOL-5-AMINE-->5-(2-ETHOXYPHENYL)-1-METHYL-3-N-PROPYL-1,6-DIHYDRO-7H-PYRAZOLO[4,3-D]-7-PYRIMIDINONE-->Antioxidant 1010-->4-CYANO-4-(4-FLUOROPHENYL)CYCLOHEXANONE-->1-(3-Hydrazinylpropyl)pyrrolidin-2-one-->1-(3-(4-AMINO-2,6-DICHLOROPHENOXY)PROPYL)PYRROLIDIN-2-ONE-->1-ethynyl-4-tert-butyl-cyclohexan-1-ol-->5-METHOXYBENZOFURAN-2-CARBOXYLIC ACID, ETHYL ESTER-->1-Ethynyl-1-cyclohexanol-->2-(DIPHENYLPHOSPHINO)ETHYLTRIETHOXYSILANE-->5-NITRO-2-HYDROXY-4-METHOXYPYRIDINE-->9-Ethyl-3-nitrocarbazole-->ETHYL 5-NITROBENZOFURAN-2-CARBOXYLATE-->4-NITRO-PYRIDIN-3-YLAMINE-->5-Methoxybenzofuran-2-carboxylic acid-->5-CHLOROBENZOFURAN-2-CARBOXYLIC ACID-->2-[2-(DIPHENYLPHOSPHINO)ETHYL]PYRIDINE-->quinuclidine-3-carboxylic acid-->5-NITROBENZOFURAN-2-CARBOXYLIC ACID-->5-CHLORO-BENZOFURAN-2-CARBOXYLIC ACID ETHYL ESTER-->Sertraline hydrochloride-->Ethyl 2-oxocyclopentanecarboxylate-->PYRIDINE-2,3,6-TRIAMINE-->1R-cis crysanthemic acid-->4-Morpholinophenylboronic acid-->ETHYL 7-METHOXYBENZOFURAN-2-CARBOXYLATE-->6-Chlorooxindole-->2,4-DICHLORO-5-NITROPYRIDINE-->Tacalcitol-->Rimonabant hydrochloride-->Stavudine-->3-Amino-1,2-benzisoxazole-->5-N-PROPYLURACIL-->1-CHLORO-4-ISOCYANOBENZENE-->3,5-DIMETHYL-1-HEXYN-3-OL-->ISOXAZOLO[5,4-B]PYRIDIN-3-YLAMINE-->Letrozole-->4-CHLORO-8-FLUORO-5H-PYRIMIDO[5,4-B]INDOLE-->2-(ETHYLTHIO)NICOTINIC ACID-->Epoprostenol |
| Questions And Answer | Back Directory | [Strong organic alkaline]
Because of the induction effect of three methyl groups in the (CH3) 3CO-, Potassium tert-butoxide has stronger alkalinity and activity than other potassium alkoxides, thus being a very excellent catalyst. In addition, as a kind of strong alkaline, potassium tert-butoxide is widely used in the organic synthesis of chemical, pharmaceutical, and pesticide such as trans-esterification, condensation, rearrangement, polymerization, loop opening and the production of heavy metal orthoester. It can be used to catalyze the Michael addition reaction, Pinacol rearrangement reaction and Ramberg-Backlund rearrangement reaction. Potassium tert-butoxide is used as a condensing agent, which can be used to catalyze Darzens condensation reaction and Stobbe condensation reaction. It is also the most effective alkaline for traditional akloxide-haloform reaction generating dihalogenated carbene. | [chemical properties]
Potassium tert-butoxide is a strong alkaline condensing agent with its alkalinity is stronger than sodium methoxide and sodium ethoxide. At room temperature, this product is white or white-like solid powder with its chemical formula being (CH3) 3C-O-K, molecular weight being 112.21, density being 0.929, the melting point of 256-258°C and the boiling point being 275°C. It is easy to absorb moisture. It is soluble in tert-butanol with the solution being relative stable, and commonly used in the dehydrohalogenation reaction of halogenated hydrocarbon. Being exposure to air decomposition, it can be decomposed into potassium oxide and tert-butyl alcohol when coming across water. | [reaction]
Potassium tert-butoxide may be used as a base in the intramolecular cyclization of iodo arene to afford benzopyran via microwave method of synthesis.
Potassium tert-butoxide has been used as a strong base in the enantioselective synthesis of amines by transfer hydrogenation of N-(tertbutylsulfinyl)imines.
Take 2, 6, 6-trimethylcyclohexane-2-alkenyl formaldehyde to have reaction with allyl Grignard agent to generate cyclohexenyl butenol, followed by the oxidation under copper-zinc catalyst to generate cyclohexane alkenyl ketene, followed by isomerization in the alkali solution of potassium tert-butoxide, being able to generate Damascenone.
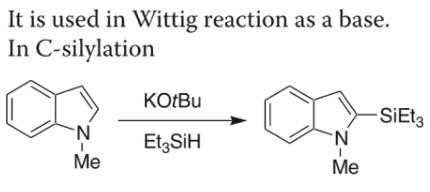
Darzen reaction: aldehydes and ketones, in the presence of alkaline reagents, can have ester-aldol condensation reaction with α-halo acid ester, while having the hydrogen halogen lost, generating α, β-epoxy ester. Common alkaline reagents include sodium ethoxide, sodium amide, sodium metal, potassium tert-butoxide and so on.
Stobbe reaction: aldehydes, ketones and succinate ester, in the presence of alkaline catalysts (such as sodium ethoxide, potassium tert-butoxide, sodium chloride, etc.) have reaction similar to the aldol condensation reaction, generating succinate or its monoester with the methylene replaced by hydrocarbon.
KOtBu: A Privileged Reagent for Electron Transfer Reactions
Potassium tert-butoxide mediated C–C, C–N, C–O and C–S bond forming reactions | [Uses]
Potassium tert-butoxide, as a strong alkaline, is widely used in the condensation, rearrangement and ring opening reaction in organic production in the fields of chemical, pharmaceutical and pesticide.
It can also be used:
To synthesize aliphatic and aromatic amides from corresponding esters and amines.
As a base in the intramolecular cyclization of aryl ethers, amines, and amides.
As a catalyst to prepare styrene derivatives from aryl halides and alkenes by Mizoroki-Heck reaction. | [preparation]
The potassium metal was added to the freshly distilled tert-butanol under nitrogen atmosphere, refluxed to until the potassium was completely melted. Further incubate for 1 hour. The excess amount of t-butyl alcohol was distilled off with the remaining white solid subjecting to vacuum drying under reduced pressure at 180-190 °C for 10 h. Then we can obtain the crystal powder of potassium tert-butanol. It needs to saved be under the nitrogen atmosphere for using. It should be kept away from air & water, or will become pink. The yield, calculated according to potassium, is more than 99%. The chemical reaction equation for the reaction between tert-butyl alcohol and metal potassium for preparation of potassium tert-butoxide is as follows:
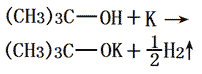 | [Precautions]
Potassium tert-butoxide has two types, liquid and solid. Usually the liquid industrial product is the tert-butanol solution of tert-butanol potassium with the color of the product being light yellow or milky white, being slightly turbid, in which the content of potassium tert-butoxide being 10% to 12%; the solid product is generally white or white powder in which the potassium tert-butoxide content is 95% to 97%.
Potassium tert-butoxide is organic alkaline corrosion product with strong moisture absorption property. It should be generally sealed for storage, generally stored in a cool, dry, ventilated warehouse. It should be kept away from heat and isolated from fire isolation as well as being protected from sun exposure. Potassium tert-butoxide has a strong corrosive effect on the skin. During the handling and loading process, the operator should wear a protective mask to prevent corrosion and burning by potassium tert-butoxide.
The above information is edited by Tongtong from Chemicalbook. |
| Hazard Information | Back Directory | [Chemical Properties]
white crystalline powder | [General Description]
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product has been enhanced for catalytic efficiency. Find details here. | [Fire Hazard]
Potassium tert-butoxide is a flammable solid.
It ignites on heating. Being very strongly
basic, its reactions with acids are highly
exothermic. Contact of solid powder with
drops of sulfuric acid and vapors of acetic
acid caused ignition after an induction period
of 0.5 and 3 minutes, respectively (Manwaring
1973). Ignition occurs upon reactions
with many common solvents of the
type ketone, lower alcohols, esters, and halogenated
hydrocarbons. Such solvents include
acetone, methyl ethyl ketone, methyl isobutyl
ketone, methanol, ethanol, n-propanol, isopropanol,
ethyl acetate, n-propyl formate,
n-butyl acetate, chloroform, methylene chloride,
carbon tetrachloride, epichlorohydrin,
dimethyl carbonate, and diethyl sulfate
(NFPA 1997). Such ignition may arise from
accidental contact of the alkoxide with these
solvents and may be attributed to sudden
release of energy from exothermic reactions.
However, slow mixing of the powder
with excess solvent will dissipate the
heat. It reacts violently with water, producing
tert-butanol and potassium hydroxide, as
follows:
K―OC―(C4H9)3+H2O →
tert-C4H9OH +KOH
The addition of potassium tert-butoxide
to the solvent dimethyl sulfoxide can cause
ignition of the latter (Bretherick 1995). | [Flammability and Explosibility]
Flammable | [Purification Methods]
It sublimes at 220o/1mm. The last traces of tert-BuOH are removed by heating at 150-160o/2mm for 1hour. It is best prepared afresh as likely impurities are tert-BuOH, KOH and K2CO3 depending on its exposure to air. Its solubility at 25-26o in hexane, toluene, Et2O, and THF is 0.27%, 2.27%, 4.34% and 25.0%, respectively. [Feuer et.al. J Am Chem Soc 78 4364, Doering & Urban J Am Chem Soc 78 5938 1956, Beilstein 1 IV 1612.] |
|
|