| Identification | More | [Name]
Ganciclovir | [CAS]
82410-32-0 | [Synonyms]
2-AMINO-1,9-[[2-HYDROXY-1-(HYDROXYMETHYL)ETHOXY]METHYL]6H-PURIN-6-ONE
2-amino-1,9-dihydro-9-((2-hydroxy-1-(hydroxymethyl)ethoxy)methyl)-6h-purin-6-one
2-AMINO-9-(2-HYDROXY-1-HYDROXYMETHYL-ETHOXYMETHYL)-1,9-DIHYDRO-PURIN-6-ONE
2'-ndg
2'-nor-2'-deoxyguanosine
9-[(1,3-DIHYDROXY-2-PROPOXY)METHYL]GUANINE
9-((2-hydroxy-1-(hydroxymethyl)ethoxy)-methyl)guanine
AKOS NCG1-0049
GANCICLOVIR
GANCYCLOVIR
6h-purin-6-one,2-amino-1,9-dihydro-9-((2-hydroxy-1-(hydroxymethyl)ethoxy)met
9-((2-hydroxy-1-(hydroxymethyl)ethoxy)methyl)-guanin
biolf62
biolf-62
bw-759
bw-759u
bwb759u
cymevan
cymevene
rs-21592 | [EINECS(EC#)]
627-054-3 | [Molecular Formula]
C9H13N5O4 | [MDL Number]
MFCD00870588 | [Molecular Weight]
255.23 | [MOL File]
82410-32-0.mol |
| Chemical Properties | Back Directory | [Appearance]
White Powder | [Melting point ]
250°C | [Boiling point ]
398.46°C (rough estimate) | [density ]
1.3559 (rough estimate) | [refractive index ]
1.7610 (estimate) | [Fp ]
9℃ | [storage temp. ]
2-8°C
| [solubility ]
0.1 M HCl: 10 mg/mL
| [form ]
powder
| [pka]
9.33±0.20(Predicted) | [color ]
white
| [Water Solubility ]
3.6g/L(25 ºC) | [Usage]
An antiviral | [ε(extinction coefficient)]
12.0 at 256nm at 1mM | [Merck ]
14,4363 | [InChI]
InChI=1S/C9H13N5O4/c10-9-12-7-6(8(17)13-9)11-3-14(7)4-18-5(1-15)2-16/h3,5,15-16H,1-2,4H2,(H3,10,12,13,17) | [InChIKey]
IRSCQMHQWWYFCW-UHFFFAOYSA-N | [SMILES]
N1C2=C(N=C(N)NC2=O)N(COC(CO)CO)C=1 | [CAS DataBase Reference]
82410-32-0(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
T | [Risk Statements ]
R60:May impair fertility.
R61:May cause harm to the unborn child. | [Safety Statements ]
S53:Avoid exposure-obtain special instruction before use .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) . | [RIDADR ]
UN1230 - class 3 - PG 2 - Methanol, solution | [WGK Germany ]
3
| [RTECS ]
MF8407000
| [HS Code ]
34021300 | [Hazardous Substances Data]
82410-32-0(Hazardous Substances Data) | [Toxicity]
LD50 i.p. in mice: 1-2 g/kg (Martin) |
| Questions And Answer | Back Directory | [Nucleoside antiviral drugs]
Ganciclovir (ganciclovir), the chemical name 9-(1, 3-dihydroxy-2-propoxymethyl) guanine, belongs to the nucleoside antiviral drugs, being a kind of guanosine derivatives. It has broad-spectrum, high-efficient inhibitory effects on herpes virus and is the first-choice drug for the treatment of cytomegalovirus infection with strong effect on hepatitis B virus and adenovirus as well. It is homologue of acyclovir (ACV) with its antiviral effect being similar as, but stronger than aciclovir, having especially strong inhibitory effects on cytomegalovirus associated with AIDS patients. It is clinical used for the treatment of induction phase and maintenance phase on immunocompromised patients (including AIDS patients) with concurrent cytomegalovirus retinitis. It can also be used for the prevention of cytomegalovirus disease for patients receiving organ transplantation or AIDS patients with positive results in cytomegalovirus serology test.
This product is a highly efficient, low-toxicity and high-selectivity virus inhibitor developed by the Syntex Company (United States). It is the first drug that has been approved by the US FDA for the treatment of cytomegalovirus (CMV) infection. Syntex has been granted of the exclusive right of production. In June 1988, this tablet has been approved for the first time to be listed in the UK, followed by being successively approved by France, the United States, Japan, and West Germany, Italy and Canada and other countries. Until the end of June 1999, it has been approved in more than 70 countries and regions for the prevention of immunodeficiency patients and the cytomegalovirus infection of patients of organ transplantation. In 2002, Ganciclovir tablets have obtained approval of the FDA, becoming available now. | [Acyclovir]
Aciclovir belongs to the second generation of broad-spectrum antiviral drugs, first developed successfully by the British Glaxo • Weier Kang (G • W) company. It first entered into market in 1981, becoming the world's first specific anti-herpes virus A ring-opening nucleoside drug that selectively blocks viral replication in cytomegalovirus (CMV)-infected cells. However, one disadvantage of acyclovir is that it may lead to the compliance problem during compound therapy of HIV patients; during clinical treatment, the anti-cytomegalovirus effect of acyclovir is poor with high doses being able to cause glomerular block. The biggest drawback is that the daily number of taking is more than the conventional dose. | [Application]
1. Induction and maintenance treatment of CMV retinitis in immunocompromised patients (including AIDS patients).
2. Prevention of cytomegalovirus infection in patients receiving organ transplants and prophylaxis of cytomegalovirus disease in HIV-positive patients with cytomegalovirus seroprevalence.
For immunodeficient patients with life-threatening or vision-related cytomegalovirus (CMV) infections, such as AIDS, exogenous immunosuppressive patients associated with organ transplantation and tumor chemotherapy.
Antiviral drugs, for the treatment of cytomegalovirus (CMV) infections caused by low immune capability.
For antiviral drugs intermediates | [Synthesis route]
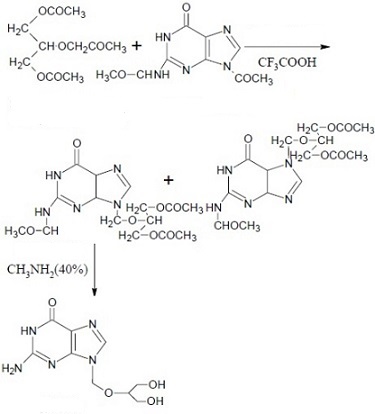
Figure 1 shows the synthesis route of ganciclovir
| [Pharmacokinetics]
Its oral absorption is poor. After fasting medication, the bioavailability is 5%. After taking the medication, the value becomes 6% to 9%. After a single round of oral administration of 3g, the peak plasma concentration can reach up to 1~1.2 mg/L with intravenous infusion of 5 mg/kg (1 hour) becoming 8.3~9 mg/L. The drug is widely distributed in the body in a variety of tissues, being able to penetrate through the placenta, but also being able to enter into the eye tissue. In the cerebrospinal fluid, the concentration is about 24% to 70% of the plasma concentration in the same period with a distribution volume of 0.74 L/kg. The protein binding rate is low (1% to 2%). The drug by intravenous administration has a half-life of 2.5 to 3.6 hours, compared with 3.1 to 5.5 hours through oral administration. Patients of renal dysfunction can be extended to 9 to 30 hours (intravenous) and 15.7 to 18.2 hours (oral). Drugs can’t be subject to metabolism, mainly being subject to renal excretion in the form of prototype, might be subject to hemodialysis or peritoneal dialysis to remove. | [Side effects]
1. A common adverse reaction is bone marrow suppression with about 40% of patients having neutropenia decreased to 1000/mm3 below and about 20% of patients having platelet count decreased to 50000/mm3 below. In addition, there could also be anemia. Hemogram is monitored throughout medication once a week.
2. Central nervous system symptoms, such as mental disorders, nervous, tremor, etc., the incidence is about 5%, occasional coma, convulsions and so on.
3 There can be rash, itch, drug fever, headache, dizziness, dyspnea, nausea, vomiting, abdominal pain, loss of appetite, abnormal liver function, gastrointestinal bleeding, arrhythmia, elevated or decreased blood pressure, hematuria, blood urea nitrogen (BUN), blood pressure, Increased, hair loss, blood sugar, edema, whole body discomfort, increased creatinine, eosinophilia, injection of local pain, phlebitis, etc.; retinal detachment may occur in AIDS patients with cytomegalovirus retinitis. | [Taboo]
Patients allergic to the goods or acyclovir should be disabled. Patients of severe neutropenia or thrombocytopenia should be disabled.
Information regarding to the application, synthesis method, pharmacokinetics and adverse reactions of ganciclovir is edited by Yan Shi from the Chemicalbook. (2015-12-02) | [Precautions]
1. This chemical structure of this product is similar to that of acyclovir. Patients allergic to the latter may also be allergic to this product.
2. This product does not cure cytomegalovirus infection, so for AIDS patients with cytomegalovirus infection often require long-term maintenance of medication, to prevent recurrence.
3. This product must be subject to intravenous administration, not intramuscular injection with each dose subjecting to at least infusion of more than 1 hour, patients need to subject to adequate amount of water, so as not to increase toxicity.
4. This product can cause neutropenia, thrombocytopenia, and easy to cause bleeding and infection, medication should pay attention to oral hygiene.
5. During the treatment, it should be always checked of the number of blood cells. During the initial treatment period, it should be measured every two days of the blood cell count, followed by changing to a weekly determination. Blood cell counts should be checked daily for patients with a history of pancytopenia, including those due to drugs, chemicals or radiation, or patients with a neutrophil count of less than 1000/mm3. When neutrophil count is below 500/mm3 or platelet count is less than 25000/mm3, it should be temporarily discontinued until the neutrophil count increased to 750/mm3 above before re-administration. For a small number of patients, it is effective for the treatment of neutropenia for granulocyte-macrophage colony-stimulating factor (GM-CSF).
6. For patients of renal dysfunction, the dose should be reduced. The dosage for hemodialysis patients per 24 hours should not exceed 1.25mg/kg. After dialysis, the plasma concentration can be reduced by about 50%, so dialysis should be administered after dialysis.
7. The pregnancy safety grading of FDA on this drug is C-class. Women of childbearing age should pay attention to the FDA should take effective contraceptive measures, men of reproductive age should be use contraceptives to at least 3 months after withdrawal.
8. For patients of AIDS combined with cytomegalovirus retinitis, during the treatment, it should be carried out of an eye examination every 6 weeks. For patients receiving zidovudine treatment, it is often can’t tolerate the joint use of this product with combination being able to cause severe leukopenia.
9. Organ transplant patients can get renal dysfunction during treatment, especially in combination with cyclosporine or amphotericin B patients. | [Medicine interactions]
1.Drugs affecting hematopoietic system, bone marrow inhibitors and radiation therapy, when used in combination with this good can enhance the inhibition of bone marrow.
2.This product, when used in combination with renal toxicity drugs at the same time (such as amphotericin B, cyclosporine) may enhance renal function damage, so that the renal excretion amount of this drug is reduced, further causing toxic reactions.
3.Combination with zidovudine or dexamethasone can enhance the toxicity to hematopoietic system, thus must be used with caution.
4.This product, when used in combination with imipenem-cilastatin can lead to the occurrence of generalized convulsions.
5. When used in combination with probenecid or drugs inhibiting the renal tubular secretion can reduce the renal clearance of this drug by about 22%, its drug-time area under the curve can increase by about 53%, and thus prone to lead to toxicity.
6 it should be avoided being used in combination with dapsone, pentamidine, flucytosine, vinblastine, doxorubicin, trimethoprim, sulfonamides and nucleoside drugs. | [Chemical properties]
It is crystallized from methanol with the melting point of 250 ° C (decomposition); it has been also reported of getting hydrate from the water with the melting point of 248-249 ° C (decomposition); crystallization from water, melting point> 300 ° C UV maximum absorption (methanol): 254nm (ε12880). Solubility in water at 25 ° C: 4.3 mg/Ml at Ph = 7, the acute toxicity: LD50 mice (g/kg): Intraperitoneal injection. | [Production method]
6-chloro guanine can be taken as raw material.
Compound (I) (15 g, 0.088 mol) was suspended in 200 ml of HMDS (1, 1, 1, 3, 3, 3-hexamethyldisilizane), 1.5 g of ammonium sulfate was added and refluxed for 2 h. The reaction solution was concentrated under reduced pressure and mercuric cyanide (24 g, (0.095 mol)) and 250 ml of benzene were added to the remaining yellow solid and the mixture was heated to reflux. A solution of 6-chloroguanine (29.93 g, 0.093 mol) in 250 mL of benzene was added and refluxed under nitrogen for 3 h. The benzene was distilled off under reduced pressure and the residue was stirred with 1.5 L of methylene chloride and filtered. The filtrate was washed twice with 300 ml of 30% potassium iodide aqueous solution, twice with 10% potassium carbonate, twice with 300 ml of water, and with 300 ml of saturated sodium chloride and dried over anhydrous sodium sulfate. Then perform concentration under reduced pressure to obtain 55 g of the crude product of the compound (II). The crude product was dissolved in a minimum amount of dichloromethane and chromatographed on a silica gel column (8.5 cm x 30 cm) eluting first with 2 L of ethyl acetate-hexane (2: 3), then further elute with 3 L of ethyl acetate-hexane 75:25). The compound (II) will be eluted from the second eluent, giving 32 g of the compound (II) with a yield of 80% yield and m.p. of 70-75 ° C.
The compound (II) (24g, 0.05mo1) was dissolved in 500ml of methanol, and a solution containing 4.6g of sodium in 200ml of methanol was added, followed by addition of 16 ml of 2-mercaptoethanol and 1ml of water and refluxed inside the nitrogen gas for 1 h. A solution of 3 g sodium dissolved in 50 ml methanol was added and refluxed for 1 h. The reaction solution was concentrated to about 70 ml under reduced pressure, poured into 400 ml of water, and adjusted to pH 6 with glacial acetic acid. The precipitate precipitated was collected by filtration, washed thoroughly with water, washed with ether and dried in vacuo to give 22 g of a solid. The solid was stirred with hot ethyl acetate and the solid was collected by filtration and recrystallized from absolute ethanol to give 16 g of compound (III) with a yield of 70% yield and m.p. of 181-183C.
Compound (m) (15.6 g, 36 mmol) was dissolved in 800 ml of refluxing ethanol. 400 ml of cyclohexene and 15 g of platinum black were added and refluxed for 18 h. The catalyst was removed by filtration and washed 5 times with hot dimethylformamide (200 ml, 100 ° C). The washings and filtrate were combined and concentrated. The residue was dissolved in hot ethanol-water (200 ml each) and filtered through 0.5 g of activated charcoal. The filtrate was concentrated under reduced pressure and the residue was crystallized from ethanol-water (4: 1) to give 7.72 g of ganciclovir in a yield of 84%, mp> 285C (decomp). |
| Hazard Information | Back Directory | [Description]
Ganciclovir is a parenterally-active antiviral agent indicated for sight- or life-threatening
cytomegalovirus (CMV) infections in immunocompromised patients. Its suppressive
effects on bone marrow and renal tubular secretion/absorption are reported to present
potential limitations on adjunct therapies involving zidovudine, vincristine, adriamycin
and amphotericin B. Recently, the emergence of CMV strains resistant to ganciclovir
therapy has been reported. | [Originator]
Enscor (United Kingdom) | [Uses]
antiemetic | [Uses]
Ganciclovir is a nucleoside analog structurally related to Acyclovir (A192400). Ganciclovir is an antiviral. | [Definition]
ChEBI: An oxopurine that is guanine substituted by a [(1,3-dihydroxypropan-2-yl)oxy]methyl group at position 9. Ganciclovir is an antiviral drug used to treat or prevent AIDS-related cytomegalovirus infections. | [Indications]
Ganciclovir (Cytovene) is an acyclic analogue of 2 deoxyguanosine
with inhibitory activity toward all herpesviruses,
especially CMV. | [Manufacturing Process]
Sodium hydride (100 g (50% dispersion in mineral oil), 2.08 mol) was washed
twice with 1 L of hexane then dried under nitrogen. Dry DMF (1.5 L) was
added. Benzyl alcohol (400 ml) was then added at for 2 hours such a rate to
keep the temperature below 50°C. Epichlorohydrin (92.5 g, 1 mol) was then
added dropwise over 0.5 hour with ice cooling in order to keep the
temperature below 40°C. The solution was next stirred for 16 hours at 21°C
then for 2.5 hours at 50°C. DMF was then removed by evaporation at reduced
pressure. The oily residue was dissolved in 2.5 L diethyl ether. The organic
solution was washed with 2 L of water, 2 L of 2% hydrochloric acid, 2 L of 1%
sodium bicarbonate, and 1 L of brine, dried over sodium sulfate, and
concentrated to a brown oil. Distillation gave 147.8 g of 1,3-di-O�benzylglycerol (boiling point 170-180°C/1 torr).
Dry hydrogen chloride gas was bubbled for 1.5 hours into a solution of 1,3-di�O-benzylglycerol (15 g, 55 mmole) and paraformaldehyde (3.3 g, 110 mmol)
in 175 ml of 1,2-dichloroethane at 0°C. The solution was then stored in a
stoppered flask for 21 hours at 4°C. Next, the solution was dried over
magnesium sulfate with warming to 21°C and then filtered and concentrated
to give 17.5 g of 1,3-di-O-benzyl-2-O-chloromethylglycerol.
To a solution of 1,3-di-O-benzyl-2-O-chloromethylglycerol (17.5 g, 55 mmol)
in 400 ml of DMF at 0°C under a drying tube was added sodium acetate (6 g).
The solution was then warmed to 21°C and magnetically stirred for 15 hours.
The solvent was removed by evaporation at reduced pressure and the oily
residue dissolved in 1 pound of diethylether. The ether solution was washed
once with 750 ml of water, two times with 250 ml of water, and once with 250
ml of brine, dried over sodium sulfate and concentrated to give 19 g of 2-O�acetoxymethyl-1,3-di-O-benzylglycerol as an oil.
Guanine (20 g, 0.132 mol) was combined with 300 ml of acetic anhydride and
the mixture heated at reflux for 16 hours. The mixture was cooled and the
excess acetic anhydride removed by evaporation at reduced pressure. The
residue was recrystallized from dimethyl sulfoxide to give 25.6 g of N2,9-
diacetylguanine.
N2,9-Diacetylguanine (15.61 g, 66 mmol), 2-O-acetoxymethyl-1,3-di-O�benzylglycerol (19 g, 55 mmol), and bis(p-nitrophenyl)phosphate (0.5 g) were
stirred together with 150 ml of diethylether. The solvent was removed by
evaporation and the residue heated in a 175°C oil bath for 1.5 hours under a
stream of nitrogen. Column chromatography eluting with 1:9
methanol/methylene chloride followed by recrystallization from ethyl acetate
afforded 4.76 g of N2,9-acetyl-9-(1,3-dibenzyloxy-2-propoxymethyl)guanine,
melting point 145-146°C.
To a solution of N2,9-acetyl-9-(1,3-dibenzyloxy-2-propoxymethyl)guanine
(4.62 g, 9.67 mmol) in 150 ml of methanol plus 40 ml of water was added
20% palladium hydroxide on carbon as a slurry in 10 ml of water. The mixture
was hydrogenated on a Parr hydrogenator at 60 psi of hydrogen for 38 hours
then filtered through celite and concentrated to a white solid. Recrystallization
from methanol/ethyl acetate gave 1.4 g of N2,9-acetyl-9-(1,3-dihydroxy-2-
propoxymethyl)guanine,melting point 205-208°C.
The mother liquor was further reduced with 10% palladium on carbon (1 g) in
150 ml of methanol plus 50 ml of water at 60 psi for 47 hours. The total yield
of N2,9-acetyl-9-(1,3-dihydroxy-2-propoxymethyl)guanine was 2.11 g.
N2,9 -Acetyl-9-(1,3-dihydroxy-2-propoxymethyl)guanine (721.9 mg, 2.4
mmol) was stirred with 50 ml of methanolic ammonia solution (methanol
saturated with ammonia at 0°C) for 17 hours at 21°C. The solution was
concentrated to a white solid and the residue recrystallized from methanol to
give 582.3 mg of 9-(1,3-dihydroxy-2-propoxymethyl)-guanine, melting point
250°C (decomp.). | [Brand name]
Cytovene (Roche); Vitrasert (Bausch & Lomb);Cymevene. | [Therapeutic Function]
Antiviral | [Acquired resistance]
Prolonged, repeated courses lead to the selection of resistant
strains, occurring in 8% of patients receiving the drug for
>3 months. Studies of laboratory-derived resistant strains
indicate that drug resistance can result from alterations in the
phosphonotransferase encoded by the gene region UL 27, the
viral DNA polymerase (gene region UL 54), or both. | [General Description]
Ganciclovir, 9-[(1,3-dihydroxy-2-propoxy) methyl]guanine)or DHPG (Cytovene), is an analog of acyclovir, with an additional hydroxymethyl group on the acyclic side chain.
After administration, similar to acyclovir, ganciclovir isphosphorylated inside the cell by a virally encoded proteinkinase to the monophosphate.Host cell enzymes catalyzethe formation of the triphosphate, which reaches more than10-fold higher concentrations in infected cells than in uninfectedcells.
The clinical usefulness of ganciclovir is limited by thetoxicity of the drug. Ganciclovir causes myelosuppression,producing neutropenia, thrombocytopenia, and anemia.These effects are probably associated with inhibition of hostcell DNA polymerase.Potential central nervous systemside effects include headaches, behavioral changes, and convulsions.Ganciclovir is mutagenic, carcinogenic, and teratogenicin animals. | [Pharmaceutical Applications]
A synthetic 2′-deoxyguanosine nucleoside analog, supplied as
the l-valine ester, valganciclovir, for oral administration and
as the sodium salt for parenteral use. A slow-release ocular
implant device is also available. | [Biochem/physiol Actions]
Cell permeable: yes | [Mechanism of action]
Ganciclovir sodium is an acyclic deoxyguanosine analogue of acyclovir. Ganciclovir inhibits DNA
polymerase. Its active form is ganciclovir triphosphate, which is an inhibitor of viral rather than of cellular
DNA polymerase. The phosphorylation of ganciclovir does not require a virus-specific thymidine kinase
for its activity against CMV. The mechanism of action is similar to that of acyclovir; however, ganciclovir
is more toxic than acyclovir to human cells. | [Pharmacology]
Activation of
ganciclovir first requires conversion to ganciclovir
monophosphate by viral enzymes: protein kinase
pUL97 in CMV or thymidine kinase in HSV. Host cell
enzymes then perform two additional phosphorylations.
The resultant ganciclovir triphosphate competes with
dGTP for access to viral DNA polymerase. Its incorporation
into the growing DNA strand causes chain termination
in a manner similar to that of acyclovir.
Ganciclovir triphosphate is up to 100-fold more concentrated
in CMV-infected cells than in normal cells and is
preferentially incorporated into DNA by viral polymerase.
However, mammalian bone marrow cells are
sensitive to growth inhibition by ganciclovir. | [Clinical Use]
Intravenous ganciclovir is indicated for the treatment
of CMV retinitis in immunocompromised individuals,
including those with AIDS, and for the prevention of
CMV infection in organ transplant recipients.Oral ganciclovir
is less effective than the intravenous preparation
but carries a lower risk of adverse effects. It is Intravenous ganciclovir is indicated for the treatment
of CMV retinitis in immunocompromised individuals,
including those with AIDS, and for the prevention of
CMV infection in organ transplant recipients.Oral ganciclovir
is less effective than the intravenous preparation
but carries a lower risk of adverse effects. It is | [Clinical Use]
Life- or sight-threatening CMV infections in immunocompromised individuals
Prevention and treatment of CMV disease in patients receiving
immunosuppressive therapy for organ transplantation
An ocular implant has been developed for the treatment of
CMV retinitis.
Use in congenital CMV infections has not yet gained regulatory
approval. | [Drug interactions]
Potentially hazardous interactions with other drugs
Antibacterials: increased risk of convulsions with
imipenem/cilastatin.
Antivirals: possibly increased didanosine
concentration; profound myelosuppression with
zidovudine - avoid if possible.
Increased risk of myelosuppression with other
myelosuppressive drugs.
Mycophenolate: concomitant treatment with
ganciclovir and mycophenolate causes increased
concentration of ganciclovir and inactive
mycophenolate metabolite. | [Metabolism]
Renal excretion of unchanged drug by glomerular
filtration and active tubular secretion is the major route of
elimination of ganciclovir. In patients with normal renal
function, 89.6 ± 5.0% of IV administered ganciclovir was
recovered unmetabolised in the urine. | [Purification Methods]
Recrystallise gangcyclovir from MeOH. Alternatively dissolve �90g of it in 700mL of �2O, filter and cool (ca 94% recovery). UV: max in MeOH 254nm (� 12,880), 270sh nm (� 9,040); its solubility in �2O at 25o is 4.3mg/mL at pH 7.0. ANTIVIRAL. [Ogilvie et al. Can J Chem 60 3005 1982, Ashton et al. Biochem Biophys Res Commun 108 1716 1982, Martin et al. J Med Chem 26 759 1983.] | [References]
[1] ding z1, mathur v, ho pp, james ml, lucin km, hoehne a, alabsi h, gambhir ss, steinman l, luo j, wyss-coray t. antiviral drug ganciclovir is a potent inhibitor of microglial proliferation and neuroinflammation. j exp med. 2014 feb 10; 211(2):189-98. |
|
|