| Identification | More | [Name]
Ferric nitrate nonahydrate | [CAS]
7782-61-8 | [Synonyms]
FERRIC NITRATE
FERRIC NITRATE 9H2O
FERRIC NITRATE, 9-HYDRATE
FERRIC NITRATE, HYDRATED
FERRIC NITRATE NONAHYDRATE
IRON(+3)NITRATE ENNEAHYDRATE
IRON (III) NITRATE
IRON(III) NITRATE-9-HYDRATE
IRON(III) NITRATE ENNEAHYDRATE
IRON (III) NITRATE, HYDROUS
IRON(III) NITRATE NONAHYDRATE
IRON(III) NITRATE, NONOHYDRATE
Iron(III)nitrate,nonahydrate(1:3:9)
nitricacid,iron(3+)salt,nonahydrate
Ironnitratenonahydratecolorlesspalevioletxtl
FERRIC NITRATE NONAHYDRATE CELL CULTURE TESTED
FERRIC NITRATE NONAHYDRATE, ACS, 98-101 %
IRON(III) NITRATE NONAHYDRATE, 99.99+%
IRON(III) NITRATE NONAHYDRATE, 98+%, A.C .S. REAGENT
Iron(III) nitrate nonahydrate, 99.999+% metals basis | [EINECS(EC#)]
233-899-5 | [Molecular Formula]
FeH18N3O18 | [MDL Number]
MFCD00149708 | [Molecular Weight]
404 | [MOL File]
7782-61-8.mol |
| Chemical Properties | Back Directory | [Appearance]
Pale purple solid | [Melting point ]
47 °C(lit.)
| [Boiling point ]
125°C | [bulk density]
900kg/m3 | [density ]
1,68 g/cm3 | [Fp ]
125°C | [storage temp. ]
Store at RT. | [solubility ]
very soluble in ethanol, acetone | [form ]
Solid | [color ]
White to gray or light purple | [Specific Gravity]
1.684 | [PH]
1.3 (100g/l, H2O, 20℃) | [Stability:]
Stable. Strong oxidizer-contact with combustible material may lead to fire. Incompatible with combustible material, strong reducing agents, most common metals, organic material. May be light sensitive. | [Water Solubility ]
soluble | [Sensitive ]
Moisture Sensitive | [Merck ]
14,4027 | [Exposure limits]
ACGIH: TWA 1 mg/m3
NIOSH: TWA 1 mg/m3 | [CAS DataBase Reference]
7782-61-8(CAS DataBase Reference) | [EPA Substance Registry System]
7782-61-8(EPA Substance) |
| Questions And Answer | Back Directory | [Description]
Iron(III) Nitrate Nonahydrate is a highly water soluble crystalline Iron source for uses compatible with nitrates and lower (acidic) pH. All metallic nitrates are inorganic salts of a given metal cation and the nitrate anion. The nitrate anion is a univalent (-1 charge) polyatomic ion composed of a single nitrogen atom ionically bound to three oxygen atoms (Formula: NO3) for a total formula weight of 62.05. | [Uses]
Ferric nitrate nonahydrate can be used as catalyst, mordant, metal surface treatment agent, oxidant, analytical reagent, radioactive substance adsorbent.
| [analysis method]
Weigh 1~1.5g sample (accurate to 0.0001g) into a 250ml dry iodine measuring flask, and record the weight m. Add 20ml of water, 3g of potassium iodide, and 10ml of sulfuric acid solution (10%), and shake it up to dissolve completely, place in a dark place for 10-15 minutes, add about 80ml of water, titrate with sodium thiosulfate standard titration solution, and add when near the end 3ml starch indicator solution, continue to titrate until the blue color of the solution fades as the end point.
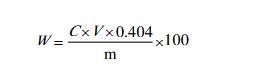
V--The value of the volume of sodium thiosulfate standard titration solution, in milliliter (mL);
c--The exact value of the concentration of sodium thiosulfate standard titration solution, the unit is moles per liter (mol/L);
0.404- -The mass of iron nitrate [(Fe (NO3) 3●9H2O)] equivalent to 1.00mL with sodium thiosulfate standard titration solution [C (Na2S2O3)=0.1000mol/L] in grams;
m---Sample mass, g.
| [Preparation]
In the iron filing method, nitric acid with a relative density of 1.38 is added to an acid-resistant reactor filled with water, heated to 40-50°C, and fine iron filings are slowly added to react for 3 hours. The solution is heated to drive out the nitrogen oxide gas. Filter, evaporate and concentrate the filtrate to form a crystal film, send it to a cooling crystallizer to cool to below 0°C to precipitate crystals. After suction filtration, the crystals are washed with 20% nitric acid. The finished Ferric nitrate nonahydrate is produced. The reaction formula is as follows. Fe+4HNO3→Fe(NO3)3+NO+2H2OFe+6HNO3→Fe(NO3)3+3NO2+3H2O
The mother liquor can be recycled. The nitrogen oxide gas released during the reaction is commonly absorbed by caustic soda solution in industry. Because of its fast absorption rate, the obtained neutralization liquid is used to produce sodium nitrate.
| [Physical Properties]
The nonahydrate form occurs as grayish-violet crystal; density 1.68 g/cm3; hygroscopic; decomposes at 47°C; very soluble in water, alcohol and acetone.
| [Uses]
Ferric nitrate nonahydrate is used as a mordant for dyeing black and buff. Other applications are in tanning; weighting silks; and in preparation of analytical standards.
| [Preparation]
Iron(III) nitrate is prepared by the action of nitric acid on iron filings or iron oxide followed by crystallization:
2Fe + 6HNO3 → 2Fe(NO3)3 + 3H2
Fe2O3 + 6HNO3 → 2Fe(NO3)3 + 3H2O
|
| Safety Data | Back Directory | [Hazard Codes ]
O,Xi | [Risk Statements ]
R8:Contact with combustible material may cause fire.
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S17:Keep away from combustible material .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing .
S24/25:Avoid contact with skin and eyes . | [RIDADR ]
UN 1466 5.1/PG 3
| [WGK Germany ]
1
| [RTECS ]
NO7175000
| [F ]
3-9-23 | [TSCA ]
Yes | [HazardClass ]
5.1 | [PackingGroup ]
III | [HS Code ]
28342980 | [Toxicity]
LD50 orally in Rabbit: 3250 mg/kg |
| Hazard Information | Back Directory | [Chemical Properties]
Pale purple solid | [General Description]
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Green Chemistry. This product has been enhanced for catalytic efficiency. Click here for more information. | [reaction suitability]
reagent type: catalyst
core: iron | [Purification Methods]
It crystallises from aqueous solutions of moderately strong HNO3 as the pale violet nonahydrate m 40o and is soluble in EtOH and Me2CO. With more concentrated aqueous solutions (containing some HNO3), the hexahydrate crystallises out m 60.5o . The anhydrous salt is slightly deliquescent and decomposes at 47o. [Lambert & Thomson J Chem Soc 97 2426 1920, Gmelin’s, Iron (8th edn) 59 Part B pp 161-172 1932.] |
|
|