| Identification | Back Directory | [Name]
(S)-2-((Methoxycarbonyl)aMino)-3-Methylbutanoic acid | [CAS]
74761-42-5 | [Synonyms]
MOC-L
MOC-Val
MOC-L-Val
Moc-Val-OH
MOC-L-Valine
Moc-L-Val-OH
Methoxycarbonyl valine
N-(methoxycarbonyl)-Valine
(methoxycarbonyl)-L-valine
N-(Methoxycarbonyl)-L-valine
L-Valine, N-(Methoxycarbonyl)-
(Synonyms:N-(methoxycarbonyl)-L-Valine)
Moc-L-Valine (N-(Methoxycarbonyl)-L-Valine)
2-Methoxycarbonylamino-3-methyl-butyric acid
(S)-2-((Methoxycarbonyl)amino)-3-methylbutanoic
N-(Methoxycarbonyl)-L-valine
(S)-2-((Methoxycarbonyl)amino)-3-methylbutanoicaci
(S)-2-[(Methoxycarbonyl)amino]-3-methylbutyric Acid
(S)-2-((Methoxycarbonyl)aMino)-3-Methylbutanoic acid
(S)-2-((Methoxycarbonyl)amino)-4-methylbutanoic Acid
(2S)-2-[(Methoxycarbonyl)aMino]-3-Methylbutanoic acid
(S)-2-((Methoxycarbonyl)aMino)-3-Methylbutanoic acid USP/EP/BP
(S)-2-((Methoxycarbonyl)aMino)-3-MethylbutaChemicalbooknoicacid | [Molecular Formula]
C7H13NO4 | [MDL Number]
MFCD12795357 | [MOL File]
74761-42-5.mol | [Molecular Weight]
175.182 |
| Chemical Properties | Back Directory | [Melting point ]
109.0 to 113.0 °C | [Boiling point ]
315.9±25.0 °C(Predicted) | [density ]
1.154±0.06 g/cm3(Predicted) | [storage temp. ]
Sealed in dry,Room Temperature | [form ]
powder to crystal | [pka]
4.44±0.10(Predicted) | [color ]
White to Almost white | [InChI]
InChI=1S/C7H13NO4/c1-4(2)5(6(9)10)8-7(11)12-3/h4-5H,1-3H3,(H,8,11)(H,9,10)/t5-/m0/s1 | [InChIKey]
CEFVHPDFGLDQKU-YFKPBYRVSA-N | [SMILES]
C(O)(=O)[C@H](C(C)C)NC(OC)=O |
| Hazard Information | Back Directory | [Uses]
(S)-2-((Methoxycarbonyl)aMino)-3-Methylbutanoic acid can be used as Ledipasvir Intermediate, mainly used in laboratory R&D process and chemical production process. | [Synthesis]
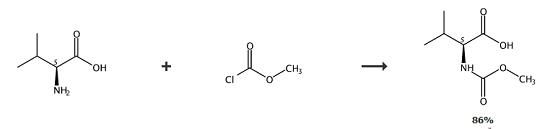
Na2CO3 (1.83 g, 17.2 mmol) was added to an aq solution of NaOH (33 mL of 1 M/H2O, 33 mmol) and L-valine (3.900 g, 33.29 mmol) and the resulting solution was cooled with ice-water bath. Methyl chloroformate (2.8 mL, 36.1 mmol) was added dropwise, the cooling bath was removed and the reaction mixture was stirred at ambient temperature for 3.25 h. The reaction mixture was washed with ether (3x17 mL), and the aqueous phase was cooled with ice-water bath and acidified with conc HCl to a pH of 1-2, and extracted with CH2Cl2 (3x17 mL). The organic phase was dried (anhyd MgSO4), filtered, and concentrated in vacuo. Cap-1 as a white solid (5.00 g, 86%). |
|
|