| Identification | Back Directory | [Name]
Ledipasvir | [CAS]
1256388-51-8 | [Synonyms]
GS5885
GS 588
CS-948
GS-5885
GS 5885
Leidipawei
Ledipasvir
332382-54-7
Ledipasvir D16
gs-5885/gs5885
Ledipasvir API
HY-15602 (GS-5885
GS-5885/Ledipasvir
Ledipasvir / GS 5885
GS 5885;GS-5885;GS5885
Ledipasvir (10mM in DMSO)
Ledipasvir (API, Amorphous)
Solid dispersion of ledipasvir
Ledipasvir undefined salt form
1H-Pyrazol-5-amine,1-(6-methylethyl)-
Ledipasvir: copovidone solid dispersion 1:1
Ledipasvir, 98%, HCV NS5A polymerase inhibitor
LedipasvirQ: What is
Ledipasvir Q: What is the CAS Number of
Ledipasvir Q: What is the storage condition of
Ledipasvir Q: What are the applications of
Ledipasvir
Ledipasvir D8Q: What is
Ledipasvir D8 Q: What is the CAS Number of
Ledipasvir D8 Q: What is the storage condition of
Ledipasvir D8 Q: What are the applications of
Ledipasvir D8
N-[(1S)-1-[[(6S)-6-[5-[9,9-Difluoro-7-[2-[(1R,3S,4S)-2-[(2S)-2-[(methoxycarbonyl)amino]-3-methyl-1-oxobutyl]-2-azabicyclo[2.2.1]hept-3-yl]-1H-benzimidazol-6-yl]-9H-fluoren-2-yl]-1H-imidazol-2-yl]-5-azaspi
Ledipasvir 13C2 D6Q: What is
Ledipasvir 13C2 D6 Q: What is the CAS Number of
Ledipasvir 13C2 D6 Q: What is the storage condition of
Ledipasvir 13C2 D6 Q: What are the applications of
Ledipasvir 13C2 D6
methyl((S)-1-((S)-6-(5-(9,9-difluoro-7-(2-((1R,3S,4S)-2-((methoxycarbonyl)-L-valyl)-2-azabicyclo[2.2.1]heptan-3-yl)-1H-benzo[d]imidazol-6-yl)-9H-fluoren-2-yl)-1H-imidazol-2-yl)-5-azaspiro[2.4]heptan-5-yl)-3-methyl-1-oxobutan-2-yl)carbamate
N-[(1S)-1-[[(6S)-6-[5-[9,9-difluoro-7-[2-[(1R,3S,4S)-2-[(2S)-2-[(methoxycarbonyl)amino]-3-methyl-1-oxoButyl]-2-azabicyclo[2.2.1]hept-3-yl]-1H-benzimidazol-6-yl]-9H-fluoren-2-yl]-1H-imidazol-2-yl]-5-azaspiro[2.4]hept-5-yl]carbonyl]-2-methylpropyl]-carbamic
N-[(1S)-1-[[(6S)-6-[5-[9,9-difluoro-7-[2-[(1R,3S,4S)-2-[(2S)-2-[(methoxycarbonyl)amino]-3-methyl-1-oxobutyl]-2-azabicyclo[2.2.1]hept-3-yl]-1H-benzimidazol-6-yl]-9H-fluoren-2-yl]-1H-imidazol-2-yl]-5-azaspiro[2.4]hept-5-yl]carbonyl]-2-methylpropyl]-carbamic acid methyl ester
Carbamic acid, N-[(1S)-1-[[(6S)-6-[5-[9,9-difluoro-7-[2-[(1R,3S,4S)-2-[(2S)-2-[(methoxycarbonyl)amino]-3-methyl-1-oxobutyl]-2-azabicyclo[2.2.1]hept-3-yl]-1H-benzimidazol-6-yl]-9H-fluoren-2-yl]-1H-imidazol-2-yl]-5-azaspiro[2.4]hept-5-yl]carbonyl]-2-methylpropyl]-, methyl ester | [EINECS(EC#)]
1592732-453-0 | [Molecular Formula]
C49H54F2N8O6 | [MDL Number]
MFCD25976756 | [MOL File]
1256388-51-8.mol | [Molecular Weight]
889 |
| Chemical Properties | Back Directory | [Melting point ]
186-190oC | [density ]
1.42±0.1 g/cm3(Predicted) | [storage temp. ]
-20°C Freezer | [solubility ]
DMSO (Slightly, Heated), Methanol (Slightly) | [form ]
Solid | [pka]
11.20±0.10(Predicted) | [color ]
White to Pale Beige |
| Hazard Information | Back Directory | [Description]
Ledipasvir is a potent NS5A inhibitor that is approved for use in
combination with sofosbuvir, a nucleotide inhibitor of viral polymerase,
for the treatment of chronic hepatitis C virus genotype 1
infection. This combination was discovered and developed
at Gilead Sciences and is marketed as the fixed combination with
brand name of Harvoni�. | [Uses]
Ledipasvir is most commonly used in combination with sofosbuvir for treatment in chronic hepatitis C genotype 1 patients. It inhibits an important viral phosphoprotein, NS5A, which is involved in viral replication, assembly, and secretion. | [Definition]
ChEBI: A benzimidazole derivative that is used in combination with sofosbuvir (under the trade name Harvoni) for the treatment of chronic hepatitis C genotype 1 infection. | [Pharmacokinetics]
Oral bioavailability of sofosbuvir is at least 80% based on urinary recovery. Ledipasvir also is well absorbed orally. Approximately 65% of sofosbuvir is bound to human plasma protein, and virtually all of ledipasvir is plasma protein bound.
The sofosbuvir Tmax is 0.5 to 2 hours, with peak plasma concentration of the active metabolite occurring 2 to 4 hours after dosing. The Tmax for ledipasvir is 4 to 4.5 hours. The terminal halflife for sofosbuvir and its active metabolite are 0.4 and 27 hours, respectively, and the terminal half-life for ledipasvir is 47 hours. Sofosbuvir primarily is eliminated in the urine, whereas ledipasvir elimination occurs primarily through the biliary tract. Eighty percent of sofosbuvir is recovered in the urine, primarily as the active metabolite, and 86% of ledipasvir is recovered in the feces. | [Synthesis]
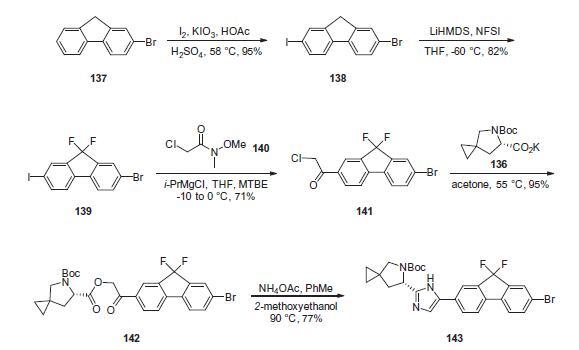
The synthesis of
the spirocyclopropane proline intermediate 136 is described in
Scheme above. Bis-iodination of cyclopropane-1,1-diyldimethanol
(131) in the presence of triphenylphosphine gave diiodide 132 in
70% yield. N-Boc-glycine ethyl ester (133) was then treated with
sodium hydride followed by diiodide 132 to give the protected proline
analog 134 in 61% yield. Saponification of the ester followed by
a classical resolution with (1S,2R)-amino-indanol gave enantomerically
pure salt 135. Liberation of the free acid with 1 M HCl followed
by treatment with potassium tert-butoxide provided
enantiopure potassium salt 136 in high yield.

Iodination of 2-bromofluorene
(137) produced aryl iodide 138 in 95% yield, which was
then treated with lithium hexamethyldisilazide and N-fluorobenzenesulfonimide
(NFSI) to give the difluoro intermediate 139 in
82% yield. Formation of the Grignard reagent of 139 through reaction
with isopropylmagnesium chloride followed by condensation
with Weinreb amide 140 gave chloroketone 141 in 71% yield. The
potassium salt of the cyclopropyl proline intermediate 136 was coupled with 141 to give keto ester
142 in high yield. Heating 142 with ammonium acetate resulted
in formation of the imidazole ring in intermediate 143 in 77%
yield.
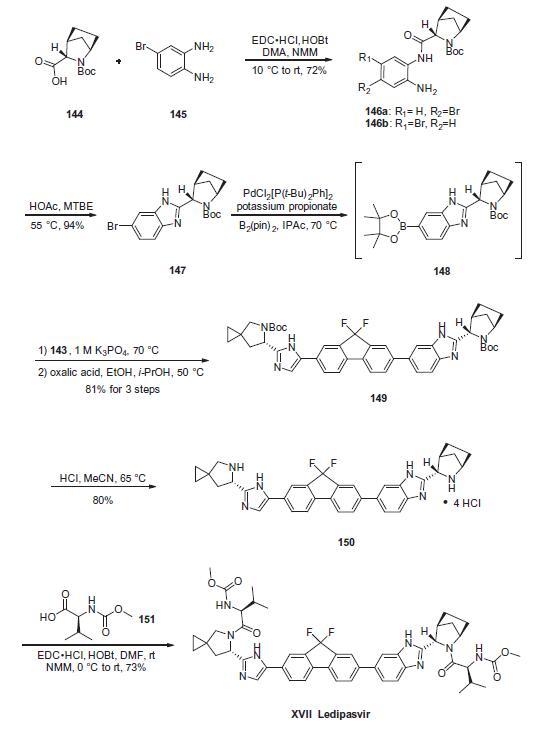
Commercially available (1R,3S,4S)-N-Boc-2-azabicyclo
[2.2.1]heptane-3-carboxylic acid (144) was coupled to 4-bromo-
1,2-benzenediamine (145) using EDC/HOBt to give a mixture of
amides 146a/146b in 72% yield. Heating mixture 146a/146b with
acetic acid affected cyclization to benzimidazole 147 in 94% yield.
Palladium mediated coupling of bromide 147 to bis(pinacolato)diboron
gave intermediate 148 which was then coupled in the same
reaction vessel to bromide 143. This was
followed by formation of the oxalate salt to give the protected central
core of ledipasvir (149) in good overall yield. Removal of the
amine protecting groups gave diamine 150 which was coupled to
two equivalents of Moc-valine (151) via EDC/HOBt to give ledipasvir
XVII in 73% yield.
 | [Clinical claims and research]
Ledipasvir is an HCV NS5A inhibitor, while sofosbuvir inhibits HCV NS5B polymerase. These two agents are combined in a fixed-dose combination tablet marked under the trade name Harvoni? for the treatment of patients with chronic HCV. A phase I study in healthy subjects demonstrated that a moderate-fat (600 kcal, 25–30% fat) or high-fat, high-calorie (1000 kcal, 50% fat) meal did not significantly alter the Cmax, AUC0-∞, or tmax of ledipasvir–sofosbuvir. A post hoc analysis of the phase III clinical trial data was performed to evaluate the effect of food on the pharmacokinetics and clinical outcomes of ledipasvir–sofosbuvir and revealed no significant effects.
Ledipasvir demonstrates pH-dependent solubility in vitro and therefore was evaluated in two phase I studies examining the effects of coadministration with a histamine H2-receptor antagonist (famotidine 40 mg) and a proton-pump inhibitor (omeprazole 20 mg). Administration of a single dose of the combination product ledipasvir–sofosbuvir with famotidine or omeprazole and food did not significantly alter the AUC or Cmax of either agent. Ledipasvir–sofosbuvir may be administered without regard to meals or timing of acid-reducing agents. | [Mode of action]
Ledipasvir is a potent inhibitor of HCV nonstructural protein 5A (NS5A), a viral phosphoprotein that plays an important but poorly understood role in viral replication, assembly, and secretion. ;Ledipasvir is approved for the treatment of genotype 1 HCV. Its safety and efficacy have not been fully established for genotypes 2 through 6. NS5A amino acid substitutions Y93H (in genotypes 1a and 1b) and Q30E (in genotype 1a) significantly reduce susceptibility to ledipasvir in cell culture and in clinical studies. Other amino acid substitutions observed in virologic treatment failures are K24R, M28T/V, Q30H/K/L (genotype 1a), and L31V/M/I (genotype 1b). Viruses with these resistance-associated mutations remained susceptible to sofosbuvir. | [References]
[1] hernandez d et al. , natural prevalence of ns5a polymorphisms in subjects infected with hepatitis c virus genotype 3 and their effects on the antiviral activity of ns5a inhibitors. j clin virol. 2013, 57(1): 13-8.
[2] gao m et al. , chemical genetics strategy identi?es an hcv ns5a inhibitor with a potent clinical effect. nature. 2010, 465: 96-100.
[3] lawitz e j et al. , a phase 1, randomized, placebo-controlled, 3-day, dose-ranging study of gs-5885, an ns5a inhibitor, in patients with genotype 1 hepatitis c. j hepatol. 2012, 57(1): 24-31. |
|
|