| Identification | More | [Name]
Pentafluoropyridine | [CAS]
700-16-3 | [Synonyms]
2,3,4,5,6-PENTAFLUOROPYRIDINE
PENTAFLUOROPYRIDINE
PERFLUOROPYRIDINE
PENTAFLUOROPYRIDINE, 99+%
Pentafluoropyridine 99%
5-Chloro-2-caynopyridine | [EINECS(EC#)]
211-839-9 | [Molecular Formula]
C5F5N | [MDL Number]
MFCD00006225 | [Molecular Weight]
169.05 | [MOL File]
700-16-3.mol |
| Chemical Properties | Back Directory | [Appearance]
CLEAR COLOURLESS LIQUID | [Melting point ]
-42 | [Boiling point ]
83-85 °C
| [density ]
1.54 g/mL at 25 °C(lit.)
| [refractive index ]
n20/D 1.386(lit.)
| [Fp ]
75 °F
| [storage temp. ]
Flammables area | [form ]
Liquid | [pka]
-12.08±0.28(Predicted) | [color ]
Clear colorless | [Specific Gravity]
1.62 | [Water Solubility ]
Not miscible or difficult to mix in water. | [BRN ]
1427064 | [InChIKey]
XTGOWLIKIQLYRG-UHFFFAOYSA-N | [CAS DataBase Reference]
700-16-3(CAS DataBase Reference) | [NIST Chemistry Reference]
Pyridine, pentafluoro-(700-16-3) |
| Questions And Answer | Back Directory | [Synthesis]
Historically, potassium fluoride has been used with solvents to perform the Halex reaction and Finger and Kruse established the use of polar aprotic solvents in the Halex reaction, with DMSO and DMF being used for the conversion of pentachloropyridine to pentafluoropyridine.
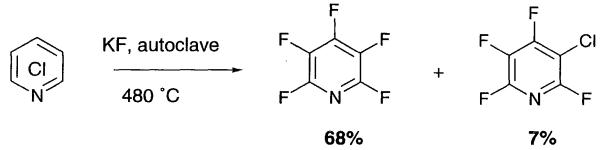 |
| Safety Data | Back Directory | [Hazard Codes ]
Xn,F,Xi | [Risk Statements ]
R10:Flammable.
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed .
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S28:After contact with skin, wash immediately with plenty of ... (to be specified by the manufacturer) .
S24/25:Avoid contact with skin and eyes .
S16:Keep away from sources of ignition-No smoking . | [RIDADR ]
UN 1993 3/PG 3
| [WGK Germany ]
3
| [Hazard Note ]
Flammable/Irritant | [HazardClass ]
3 | [PackingGroup ]
III | [HS Code ]
29333990 |
| Raw materials And Preparation Products | Back Directory | [Raw materials]
2,3,5,6-tetrachloro-4-fluoropyridine-->3-Chloro-2,4,5,6-tetrafluoropyridine | [Preparation Products]
3,5-DIFLUORO-PYRIDIN-4-YLAMINE-->Pyrrolidine, 2,2,3,3,4,4,5,5-octafluoro-1-(trifluoromethyl)--->3,5-DIFLUOROPYRIDINE-4-CARBALDEHYDE-->3,4-Dichloro-2,5,6-trifluoropyridine-->3,5-Difluoropyridine-->2,3,5,6-Tetrafluoropyridine-->2,5-Difluoropyridine-->2,3,5,6-TETRAFLUORO-4-PYRIDINE-CARBONITRILE-->2,3,4,6-TETRAFLUOROPYRIDINE-->3,4,5-Trifluoropyridine-->BENZOYL FLUORIDE-->2,3,4,5-Tetrafluoropyridine |
| Hazard Information | Back Directory | [Chemical Properties]
CLEAR COLOURLESS LIQUID | [Uses]
Pentafluoropyridine was used in the preparation of η2-C,C coordinated pentafluoropyridine complex. It was also used in the preparation of polyfluorinated coupling products via Pd(0)-catalyzed cross-coupling reaction with diarylzinc compounds. It was also used as derivatizing reagent in the sensitive GC-MS method for determination of the four endocrine disrupting chemicals. | [General Description]
The reactions of pentafluoropyridine with cobalt(0) complex, Co(PMe3)4 was investigated. | [Purification Methods]
Distil it through a concentric tube column; it has in cyclohexane at 256.8nm. [Chambers et al. J Chem Soc 3573 1964, NMR: Bell et al. J Fluorine Chem 1 51 1971.] The hexafluoroantimonate has m 98-102o(dec) after crystallisation from liquid SO2. [Beilstein 20/5 V 401.] |
|
|