| Identification | Back Directory | [Name]
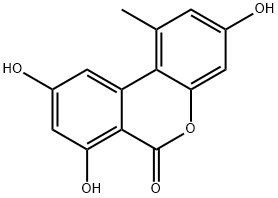
3,7,9-TRIHYDROXY-1-METHYL-6H-DIBENZO[B,D]PYRAN-6-ONE | [CAS]
641-38-3 | [Synonyms]
aoh
NSC 638263
ALTERNARIOL
Alternariol/AOH
Alternariol 99%
Alternariol 50ug/ml in ACN
alternariol from alternaria sp.
3,7,9-Trihydroxy-1-methyl-benzo[c]chromen-6-one
3,7,9-trihydroxy-1-Methyl-6H-benzo[c]chroMen-6-one
DIBENZO(B,D)PYRAN-6-ONE,1-METHYL-3,7,9-TRIHYDROXY-
d)pyran-6-one,1-methyl-3,7,9-trihydroxy-6h-dibenzo(
1-Methyl-3,7,9-trihydroxy-6H-dibenzo[b,d]pyran-6-one
3,7,9-TRIHYDROXY-1-METHYL-6H-DIBENZO[B,D]PYRAN-6-ONE
6H-Dibenzo[b,d]pyran-6-one,3,7,9-trihydroxy-1-methyl-
Alternariol 3,7,9-Trihydroxy-1-Methyl-6H-Dibenzo[b,d]Pyran-6-One
Alternariol from Alternaria sp.,3,7,9-Trihydroxy-1-methyl-6H-dibenzo[b,d]pyran-6-one | [Molecular Formula]
C14H10O5 | [MDL Number]
MFCD00133068 | [MOL File]
641-38-3.mol | [Molecular Weight]
258.23 |
| Chemical Properties | Back Directory | [Melting point ]
350°C (rough estimate) | [Boiling point ]
321.48°C (rough estimate) | [density ]
1.2211 (rough estimate) | [refractive index ]
1.4560 (estimate) | [storage temp. ]
2-8°C | [solubility ]
≤0.5mg/ml in ethanol;30mg/ml in DMSO;30mg/ml in dimethyl formamide | [form ]
neat | [pka]
7.16±0.20(Predicted) | [color ]
Off-white to brown | [BRN ]
244839 | [Stability:]
Hygroscopic |
| Safety Data | Back Directory | [Hazard Codes ]
T+ | [Risk Statements ]
26/27/28 | [Safety Statements ]
28-36/37/39-45 | [RIDADR ]
UN 3462 6.1/PG 1
| [WGK Germany ]
3
| [RTECS ]
HP8757000
| [HazardClass ]
6.1(b) | [PackingGroup ]
III | [HS Code ]
29322090 | [Toxicity]
LDLo intraperitoneal in mouse: 100mg/kg |
| Hazard Information | Back Directory | [Description]
Alternariol is a mycotoxin, a toxic secondary fungal metabolite, produced by Alternaria molds. It is cytotoxic, fetotoxic, teratogenic, mutagenic, and genotoxic. It induces cytochrome P450 1A1 expression and apoptosis in mouse hepatoma cells (20-40 μM). Alternariol, whose synthesis is inhibited by light, naturally occurs on fruits, vegetables, and cereals, such as apples, tomatoes, and wheat. | [Uses]
An alternaria mycotoxin and genotoxin, found in common edible crops. It inhibits the activity of various DNA-topoisomerases, increasing he rate of DNA strand breaks. | [Definition]
ChEBI: A benzochromenone that is 6H-benzo[c]chromen-6-one which is substituted by a methyl group at position 1 and by hydroxy groups at positions 3, 7, and 9. It is the most important mycotoxin produced by the black mould Alt
rnaria species, which are the most common mycoflora infecting small grain cereals worldwide. | [Synthesis Reference(s)]
The Journal of Organic Chemistry, 70, p. 3275, 2005 DOI: 10.1021/jo050075r | [General Description]
Alternariol belongs to the dibenzo-pyrones chemical group. It is a mycotoxin present in indoor air, soil and plants. | [Biochem/physiol Actions]
Alternaria mycotoxins are generally associated with undried food grains post-harvest. The disease caused by Alternaria mycotoxin results in a discolored halo in fruits and spotting in leaves. Alternariol (AOH) is cytotoxic and fetotoxic to microbes and mammalian cells. AOH displays inhibitory effect on cell proliferation and has estrogenic functionality. | [storage]
Store at -20°C,protect from light | [References]
[1] tiemann u, tomek w, schneider f, et al. the mycotoxins alternariol and alternariol methyl ether negatively affect progesterone synthesis in porcine granulosa cells in vitro[j]. toxicology letters, 2009, 186(2): 139-145.
[2] sderhll k, svensson e, unestam t. light inhibits the production of alternariol and alternariol monomethyl ether in alternaria alternata[j]. applied and environmental microbiology, 1978, 36(5): 655-657.
[3] schreck i, deigendesch u, burkhardt b, et al. the alternaria mycotoxins alternariol and alternariol methyl ether induce cytochrome p450 1a1 and apoptosis in murine hepatoma cells dependent on the aryl hydrocarbon receptor[j]. archives of toxicology, 2012, 86(4): 625-632. |
|
| Company Name: |
BioBioPha Co., Ltd.
|
| Tel: |
0871-65217109 13211707573; |
| Website: |
http://www.biobiopha.com |
|