| Identification | More | [Name]
5-Methoxytryptamine | [CAS]
608-07-1 | [Synonyms]
2-(5-METHOXY-1H-INDOL-3-YL)ETHANAMINE
2-(5-METHOXY-1H-INDOL-3-YL)ETHANAMINE HYDROCHLORIDE
2-(5-METHOXY-1H-INDOL-3-YL)-ETHYLAMINE
2-[5-METHOXYINDOL-3-YL]ETHYLAMINE HYDROCHLORIDE
3-(2-AMINOETHYL)-5-METHOXYINDOLE
3-[2-AMINOETHYL]-5-METHOXYINDOLE HYDROCHLORIDE
5-METHOXYTRYPTAMINE
5-METHOXYTRYPTAMINE HCL
5-METHOXYTRYPTAMINE HYDROCHLORIDE
AKOS B028531
AKOS BB-5620
AKOS JY2083095
ART-CHEM-BB B028531
AURORA KA-7772
BIO-FARMA BF000967
METHOXYTRYPTAMINE, 5-
MEXAMINE
MEXAMINE HYDROCHLORIDE
O-METHYLSEROTONIN
O-METHYLSEROTONIN HYDROCHLORIDE | [EINECS(EC#)]
200-637-6 | [Molecular Formula]
C11H14N2O | [MDL Number]
MFCD00005662 | [Molecular Weight]
190.24 | [MOL File]
608-07-1.mol |
| Chemical Properties | Back Directory | [Appearance]
White to tan crystalline powder | [Melting point ]
121-123 °C (lit.) | [Boiling point ]
325.75°C (rough estimate) | [density ]
1.0815 (rough estimate) | [refractive index ]
1.5700 (estimate) | [storage temp. ]
2-8°C
| [solubility ]
Chloroform (Slightly), Methanol (Slightly) | [form ]
crystalline
| [pka]
16.91±0.30(Predicted) | [color ]
white
| [Detection Methods]
HPLC,NMR | [Merck ]
13,6032 | [BRN ]
145587 | [InChI]
InChI=1S/C11H14N2O/c1-14-9-2-3-11-10(6-9)8(4-5-12)7-13-11/h2-3,6-7,13H,4-5,12H2,1H3 | [InChIKey]
JTEJPPKMYBDEMY-UHFFFAOYSA-N | [SMILES]
N1C2=C(C=C(OC)C=C2)C(CCN)=C1 | [CAS DataBase Reference]
608-07-1(CAS DataBase Reference) | [NIST Chemistry Reference]
5-Methoxytryptamine(608-07-1) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R22:Harmful if swallowed.
R36/37/38:Irritating to eyes, respiratory system and skin .
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed . | [Safety Statements ]
S36:Wear suitable protective clothing .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice . | [WGK Germany ]
3
| [RTECS ]
NL4110000
| [F ]
8 | [HS Code ]
29339900 | [Toxicity]
LD50 intraperitoneal in mouse: 176mg/kg |
| Hazard Information | Back Directory | [Description]
5-Methoxytryptamine (5-MT), also known as mexamine, is a tryptamine derivative closely related to the neurotransmitters serotonin and melatonin. 5-MT has been shown to occur naturally in the body in low levels.It is biosynthesized via the deacetylation of melatonin in the pineal gland.
The protective effect of 5-methoxytryptamine (a metabolite of melatonin) in human keratinocytes against ultraviolet B (UVB) radiation was studied.
Melatonin and its metabolites ameliorate ultraviolet B-induced damage in human epidermal keratinocytes. | [Chemical Properties]
White to tan crystalline powder | [Uses]
5HT agonist | [Uses]
5-Methoxytryptamine was used as an agonist in the study of pharmacological profile of the 5-hydroxytryptamine 1 receptor.
Reactant for preparation of:
Carboline disaccharide domain of shishijimicin A
Melatonin analogs for the reduction of intraocular pressure
5-HT4 receptor ligands
inhibitors of sortase A and isocitrate lyase
Therapeutic agents for treatment of ischemia/reperfusion (I/R) injury
Aurora and epidermal growth factor receptor kinase inhibitors
Agents for the treatment of human papillomavirus infection
Manzamine analogues for the control of neuroinflammation and cerebral infections
Inhibitors of pro-inflammatory cytokines
Tacrine-melatonin hybrids as multifunctional agents for alzheimer′s disease | [Uses]
A tryptamine derivative that is closely related to the neurotransmitter Melatonin (M215000) and Serotonin (S274980). It acts as a full agonist at the 5-HT1, 5-HT2, 5-HT4, 5-HT6, and 5-HT7 receptors but has no affinity for the 5-HT3 receptor. | [Application]
5-Methoxytryptamine was used as an agonist in the study of pharmacological profile of the 5-hydroxytryptamine 1 receptor.
Reactant for preparation of:
Carboline disaccharide domain of shishijimicin A
Melatonin analogs for the reduction of intraocular pressure
5-HT4 receptor ligands
inhibitors of sortase A and isocitrate lyase
Therapeutic agents for treatment of ischemia/reperfusion (I/R) injury
Aurora and epidermal growth factor receptor kinase inhibitors
Agents for the treatment of human papillomavirus infection
Manzamine analogues for the control of neuroinflammation and cerebral infections
Inhibitors of pro-inflammatory cytokines
Tacrine-melatonin hybrids as multifunctional agents for alzheimer′s disease | [Definition]
ChEBI: A member of the class of tryptamines that is the methyl ether derivative of serotonin. | [General Description]
The protective effect of 5-methoxytryptamine (a metabolite of melatonin) in human keratinocytes against ultraviolet B (UVB) radiation was studied. | [Biochem/physiol Actions]
Nonselective serotonin receptor agonist that lacks affinity for the 5-HT3 receptor. | [Synthesis]
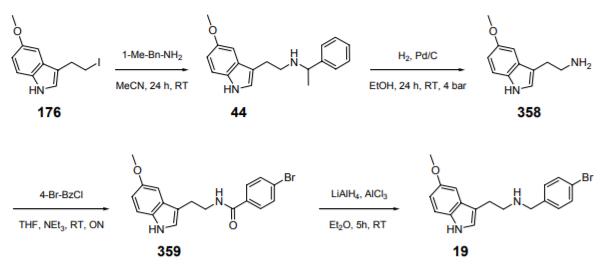
5-Methoxytryptamine (358) was synthesized from 3-(2-iodoethyl)-5-methoxyindole (176) by reaction with 1-methyl-benzylamine (MeCN, 24 h, RT) and subsequent catalytic debenzylation of 44 (H2, Pd/C, EtOH, 24 h, RT, 4 bar). The resulting 5-methoxytryptamine (358 was then reacted with 4-bromobenzoylchloride (THF, NEt3, RT, ON) and the resulting tryptamide 359 was reduced with aluminum hydride to N-(4-bromobenzyl)-5-methoxytryptamine (19) (LiAlH4, AlCl3, Et2O, 5 h, RT), which was isolated as its hydrogen oxalate salt.
 | [storage]
Store at -20°C | [Clinical claims and research]
The effects of the 5-HT receptor agonist, 5-methoxytryptamine, on plasma glucose levels were investigated in rats. 5-Methoxytryptamine induced a significant hyperglycemia above the dosage of 1 mg/kg. 5-Methoxytryptamine-induced hyperglycemia was antagonized by pretreatment with the 5-HT1 and 5-HT2 receptor antagonist, methysergide, or the 5-HT2A receptor antagonist, ketanserin, whereas the 5-HT3 and 5-HT4 receptor antagonist, tropisetron, and the 5-HT4 receptor antagonist, SDZ 205-557 (2-methoxy-4-amino-5-chloro-benzoic acid 2-(diethylamino) ethyl ester), showed no effect. In addition, the peripheral 5-HT2 receptor antagonist, xylamidine, reduced 5-methoxytryptamine-induced hyperglycemia.
Hyperglycemia induced by the 5-HT receptor agonist, 5-methoxytryptamine, in rats: involvement of the peripheral 5-HT2A receptor. |
|
|