| Identification | More | [Name]
N-Hydroxysuccinimide | [CAS]
6066-82-6 | [Synonyms]
1-HYDROXY-2,5-PYRROLIDINEDIONE
1-HYDROXY-PYRROLIDINE-2,5-DIONE
2,5-PYRROLIDINEDIONE, 1-HYDROXY-
4-HYDROXY-2,5-PYRROLIDINEDIONE
AKOS 91864
HONSU
HOSU
NHS
N-HYDROXY-2,5-PYRROLIDINEDIONE
N-HYDROXYSUCCINIMDE
N-HYDROXYSUCCINIMIDE
N-HYDROXYSUCCINIMIDE POLYMER-BOUND
1-hydroxy-5-pyrrolidinedione
1-Hydroxysuccinimide
5-Pyrrolidinedione,1-hydroxy-2
Hydroxysuccinimide
Succinimide, N-hydroxy-
HOSu~1-Hydroxy-2,5-pyrrolidinedione
N-Hydroxysuccinimide (HOSu)
N-HYDROXYSUCCINAMIDE | [EINECS(EC#)]
228-001-3 | [Molecular Formula]
C4H5NO3 | [MDL Number]
MFCD00005516 | [Molecular Weight]
115.09 | [MOL File]
6066-82-6.mol |
| Chemical Properties | Back Directory | [Appearance]
White to pale yellow | [Melting point ]
95-98 °C(lit.)
| [Boiling point ]
215.33°C (rough estimate) | [density ]
1.4769 (rough estimate) | [vapor pressure ]
0Pa at 25℃ | [refractive index ]
1.4080 (estimate) | [storage temp. ]
2-8°C | [solubility ]
DMSO (Soluble), Methanol (Slightly) | [form ]
Liquid or Solid | [pka]
7.81±0.20(Predicted) | [color ]
Clear yellow-brown to brown | [PH]
5-7 (50g/l, H2O, 20℃) | [Stability:]
Stable. Incompatible with strong oxidizing agents, acid chlorides, acid anhydrides, strong bases. Protect from moisture. | [Water Solubility ]
SOLUBLE | [Sensitive ]
Hygroscopic | [Usage]
Additive used in the carbodiimide method for improved amidations and peptide couplings.
| [Detection Methods]
T,NMR | [BRN ]
113913 | [InChIKey]
NQTADLQHYWFPDB-UHFFFAOYSA-N | [LogP]
-2.01 | [Uses]
N-hydroxysuccinimide (NHS) is often used to assist carbodiimide-mediated peptide coupling by forming an active ester intermediate via condensation of the surface carboxyl group and NHS. The NHS-reactive ester intermediate is susceptible to nucleophilic attack by primary amines and results in the formation of stable amide bonds between the biomaterial surface and the N-terminus of the peptide. | [CAS DataBase Reference]
6066-82-6(CAS DataBase Reference) | [NIST Chemistry Reference]
N-hydroxylsuccinimide(6066-82-6) | [EPA Substance Registry System]
6066-82-6(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S22:Do not breathe dust .
S24/25:Avoid contact with skin and eyes .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing . | [WGK Germany ]
3
| [Hazard Note ]
Irritant | [TSCA ]
Yes | [HS Code ]
29251995 |
| Hazard Information | Back Directory | [Description]
N-hydroxysuccinimide is a synthetic ingredient used in cosmetics as an ester to soften and condition skin. It's also a reagent, a substance used to trigger a reaction that leads to a new substance, such as peptides and polymers.
N-Hydroxysuccinimide is used in cosmetics and beauty products as an ester often seen in eye creams. N-hydroxysuccinimide activates the elimination of blood originated pigments responsible for dark color and inflammation that causes under eye circles. "Infra-orbital shadows are due to the accumulation of hemoglobin and its coloured degradation products - biliverdin, bilirubin and iron - in the dermis and epidermis ... N-hydroxysuccinimide renders the iron soluble for natural elimination" (source). | [Chemical Properties]
White to pale yellow | [Application]
N-Hydroxysuccinimide has been used in the synthesis of intermediates such as:
N-succinimidyl 3-(di-tert-butylfluorosilyl)benzoate
4-[2,2-bis[(p-tolylsulfonyl)-methyl] acetyl]benzoic acid-NHS ester
N-succinimidyl 3-iodobenzoate
It has also been used in a protocol for the surface modification of microchannels of a microfluidic-integrated surface plasmon resonance (SPR) platform for the detection and quantification of bacterial pathogens.
Additive used in the carbodiimide method for improved amidations and peptide couplings.
N-Hydroxysuccinimide can be used in the synthesis of N-hydroxysuccinimide ester via dehydration reaction in the presence of dicyclohexylcarbodiimide in carboxylic acid. | [Preparation]
The preparation of N-Hydroxysuccinimide is as follows:Adding 16.12g of chitosan and 57g of N-hydroxysuccinimidyl-4-azidobenzoate to the reactor, adding 70g of PBS buffer and 300mL of dichloromethane solvent, stirring and mixing evenly Then, put it into the microwave reactor, put the temperature sensor into the reaction liquid, then turn on the magnetic stirring, adjust the stirring speed to make the stirrer rotate stably in the solution, then close the door of the microwave reactor, and set the reaction temperature to 50 ° C, and set the reaction time is 5 hours, the reaction is carried out under microwave heating assisted conditions, the reaction time is reached after the mega-reaction time reaches the set time, the sample is confirmed to be complete, and then slowly cooled to room temperature, stirred for 30 min, suction filtration A dichloromethane solution containing N-hydroxysuccinimide was obtained, wherein 22.60 g of N-hydroxysuccinimide was obtained in a yield of 98.2%, and the purity was 99.6%.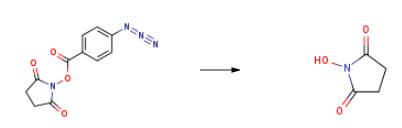
| [reaction suitability]
reaction type: Coupling Reactions | [Synthesis]
N-Hydroxysuccinimide can be synthesized by heating succinic anhydride with hydroxylamine or hydroxylamine hydrochloride. | [Purification Methods]
Recrystallise the imide from EtOH/ethyl acetate [Manesis & Goodmen J Org Chem 52 5331 1987]. [Beilstein 21/9 V 498.] |
|
|