| Identification | Back Directory | [Name]
beta-Nicotinamide adenine dinucleotide disodium salt | [CAS]
606-68-8 | [Synonyms]
NADH
DPNH
NADH2
ENADA
N 8129
NADH 2Na
NADH-NA2
DPNH2-NA2
DPNH, NA2
Co I reduced
Nadide sodium
BETA-NADH 2NA
β-NADH/β-DPNH
NADH (sodium salt)
CoenzyMe I Reduced
CozyMase I Reduced
BETA-NADH DISODIUM
NADH DISODIUM SALT
DPNH DISODIUM SALT
beta-Nicotinamide ad
Codehydrase I Reduced
BETA-NADH DISODIUM SALT
BETA-DPNH DISODIUM SALT
NADH,NADH DISODIUM SALT
Dihydrocodehydrogenase I
DihydrocozyMase DisodiuM
B-NAD, REDUCED, DISODIUM
Codehydrogenase I Reduced
Reduced Codehydrogenase I
B-NAD REDUCED FORM DISODIUM
DIPHOSPHOPYRIDINE NUCLEOTIDE
LB MODIFIED IN BOTTLES 1000ML
BIURET REAGENT WORKING SOLUTION
BETA-NAD REDUCED FORM DISODIUM
N A D H, REDUCED FORM, DISODIUM
Coenzyme I reduced disodium salt
Diphosphopyridine nucleotide reduced
Reduced Diphosphopyridine Nucleotide
Reduced NcotinaMide Adenine Diphosphate
NADH disodiuM salt, trihydrate, reduced | [EINECS(EC#)]
210-123-3 | [Molecular Formula]
C21H27N7Na2O14P2 | [MDL Number]
MFCD00036200 | [MOL File]
606-68-8.mol | [Molecular Weight]
709.4 |
| Chemical Properties | Back Directory | [Definition]
NADH, disodium salt(606-68-8) is a dehydrogenase complex that is the reduced formof NAD. As a reagent, NADH can be used in enzyme cycling assays to amplify detection of activity of biologically relevant enzymes or metabolites present in low concentrations. | [Appearance]
beige powder | [Melting point ]
140-142°C | [density ]
1.955 at 20℃ | [vapor pressure ]
0.73Pa at 20-50℃ | [storage temp. ]
2-8°C
| [solubility ]
H2O: 50 mg/mL, clear to nearly clear, yellow
| [form ]
Powder | [color ]
Yellow | [PH]
7.5 (100mg/mL in water, ±0.5) | [Stability:]
Stable. Incompatible with strong oxidizing agents. | [Water Solubility ]
soluble | [λmax]
260 and 340 nm | [BRN ]
5230241 | [InChIKey]
QRGNQKGQENGQSE-WUEGHLCSSA-L | [LogP]
-1.96 at 21℃ and pH7 | [Surface tension]
69.22mN/m at 1.022g/L and 20℃ | [CAS DataBase Reference]
606-68-8 | [EPA Substance Registry System]
Adenosine 5'-(trihydrogen diphosphate), P'.fwdarw.5'-ester with 1,4-dihydro-1-.beta.-D-ribofuranosyl- 3-pyridinecarboxamide, disodium salt(606-68-8) |
| Hazard Information | Back Directory | [Chemical Properties]
beige powder | [Uses]
One of the biologically active forms of nicotinic acid. Serves as a coenzyme of hydrogenases and dehydrogenases. NAD usually acts as a hydrogen acceptor, forming NADH which then serves as a hydrogen d
onor in the respiratory chain. Present in living cells primarily in the reduced form (NADPH) and is involved in synthetic reactions.
Occurs in 2 forms, α-NAD and β-NAD, distinguished by the configura
tion of the ribosyl nicotinamide linkage. Only the β-anomer is bioactive. | [Description]
β-Nicotinamide adenine dinucleotide (NAD+) and β-Nicotinamide adenine dinucleotide, reduced (NADH) comprise a coenzyme redox pair (NAD+:NADH) involved in a wide range of enzyme catalyzed oxidation reduction reactions. In addition to its redox function, NAD+/NADH is a donor of ADP-ribose units in ADP-ribosylaton (ADP-ribosyltransferases; poly(ADP-ribose) polymerases ) reactions and a precursor of cyclic ADP-ribose (ADP-ribosyl cyclases).
As a reagent, NADH can be used in enzyme cycling assays to amplify detection of activity of biologically relevant enzymes or metabolites present in low concentrations. | [Biological Functions]
NADH disodium salt (Disodium NADH) is an orally active reduced coenzyme. NADH disodium salt is a donor of ADP-ribose units in ADP-ribosylaton reactions and a precursor of cyclic ADP-ribose. NADH disodium salt plays a role as a regenerative electron donor in cellular energy metabolism, including glycolysis, β-oxidation and the tricarboxylic acid (TCA) cycle. | [Purification Methods]
This coenzyme is available in high purity, and it is advisable to buy a fresh preparation rather than to purify an old sample as purification will invariably lead to a more impure sample contaminated with the oxidised form (NAD). It has UV max at 340nm ( 6,200 M-1cm-1) at which wavelength the oxidised form NAD has no absorption. At 340nm a 0.161mM solution in a 1cm (pathlength) cell has an absorbance of 1.0 unit. The purity is best checked by the ratio A280nm/A340nm ~2.1, a value which increases as oxidation proceeds. The dry powder is stable indefinitely at -20o. Solutions in aqueous buffers at pH ~7 are stable for extended periods at -20o and for at least 8hours at 0o, but are oxidised more rapidly at 4o in a cold room (e.g. almost completely oxidised overnight at 4o). [UV: Drabkin J Biol Chem 175 563 1945, Fluorescence: Boyer & Thorell Acta Chem Scand 10 447 1956, Redox: Rodkey J Biol Chem 234 188 1959, Schlenk in The Enzymes 2 250, 268 1951, Kaplan in The Enzymes 3 105, 112 1960.] Deuterated NADH, i.e. NADD, has been purified through the anion exchange resin AG-1 x 8 (100-200 mesh, formate form) and through a Bio-Gel P-2 column. [Viola et al. Anal Biochem 96 334 1979, Beilstein 26 III/IV 3639.] | [General Description]
β-Nicotinamide adenine dinucleotide (β-NAD) regulates energy metabolism and immunity. It is a cofactor for mitochondrial deacetylase sirtuin-3 enzyme and modulates inflammasome assembly. β-NAD supresses interleukin-1β levels in monocytic cells in inflammatory syndromes. β-NAD released by neurosecretory cells is a potential neurotransmitter. β-NAD is a vascular mediator in lung endothelial cells and may play a protective role against cytokine mediated inflammation. | [Biochem/physiol Actions]
NADH(606-68-8) is a coenzyme that functions as a regenerating electron donor in catabolic processes including glycolysis, β-oxidation and the citric acid cycle (Krebs cycle, TCA cycle). It participates in cell signaling events as well, for example as a substrate for the poly (ADP-ribose) polymerases (PARPs) during the DNA damage response. The NAD+/NADH dependent sirtuins play key roles in stress responses during events involving energy metabolism, with implications in cancer biology, diabetes and neurodegenerative disease. | [Biotechnological Applications]
Reduced β-nicotinamide adenine dinucleotide (NADH) plays a major role in metabolism as a cofactor in redox reactions and as a mobile electron carrier. NADH is a high energy compound that donates electrons to the electron transport chain to provide energy for ATP production by oxidative phosphorylation. NADH is a required oxidizing cosubstrate in fermentation, which regenerates NAD. NADH is fluorescent, which provides for a relatively simple way to detect NADH in biological samples. NADH is also used in enzyme cycling assays to detect relevant biological molecules in tissues. | [in vitro]
NADH is unstable under acidic conditions but it is stable under alkaline conditions.
NADH (0-1 mM; 0-12 h) increases NAD levels in various mammalian cell lines+.
NADH (1 mM; 24 h) causes low toxicity and protects cells from genotoxicity.
| [in vivo]
NADH (5 μmol/mouse; i.p.; once) increases urinary excretion of nicotinamide and its metabolites in mice.
NADH (500 mg/kg; i.g.; once) promotes alcohol metabolism and prevents or ameliorates early liver injury caused by acute alcohol exposure in ethanol-loaded mice.
NADH (1000 mg/kg; i.p.; once) enhances tissue NAD levels in male C57BL/6J mice+.
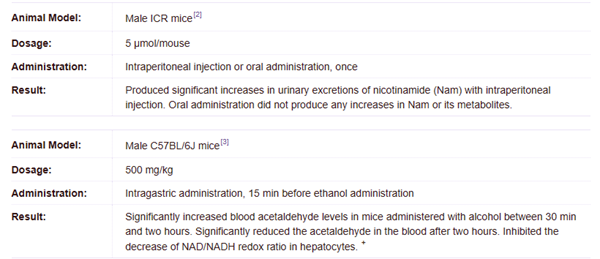
| [storage]
Store at -20°C | [References]
[2]. Kimura N, et al. Comparison of metabolic fates of nicotinamide, NAD+ and NADH administered orally and intraperitoneally; characterization of oral NADH. J Nutr Sci Vitaminol (Tokyo). 2006 Apr;52(2):142-8. [Content Brief]
[3]. Wu K, et al. NADH and NRH as potential dietary supplements or pharmacological agents for early liver injury caused by acute alcohol exposure. Journal of Functional Foods, 2021, 87: 104852. |
|
|