| Identification | More | [Name]
Nicotinamide | [CAS]
98-92-0 | [Synonyms]
3-PYRIDINECARBOXAMIDE
3-PYRIDINE CARBOXYLIC ACID AMIDE
3-PYRIDINECARBOXYLIC AMIDE
NIACINAMIDE
NICETHAMIDUM
NICOTINAMIDE
NICOTINIC ACID AMIDE
PYRIDINE-3-CARBOXAMIDE
PYRIDINE-3-CARBOXYLIC ACID AMIDE
TIMTEC-BB SBB004283
VITAMIN B3
VITAMIN B3/B5
VITAMIN PP
-(Aminocarbonyl)pyridine
3-Carbamoylpyridine
3-Pyridinecarboxyamide
Acid amide
acidamide
Amid kyseliny nikotinove
Amide PP | [EINECS(EC#)]
202-713-4 | [Molecular Formula]
C6H6N2O | [MDL Number]
MFCD00006395 | [Molecular Weight]
122.12 | [MOL File]
98-92-0.mol |
| Chemical Properties | Back Directory | [Appearance]
White crystalline powder | [Melting point ]
128-131 °C(lit.)
| [Boiling point ]
150-160 °C
| [bulk density]
360kg/m3 | [density ]
1.40 | [vapor density ]
4.22 (vs air)
| [vapor pressure ]
0Pa at 25℃ | [refractive index ]
1.4660 (estimate) | [Fp ]
182 °C
| [storage temp. ]
0-6°C | [solubility ]
691g/l | [form ]
powder
| [pka]
3.3(at 20℃) | [color ]
white
| [Odor]
Odorless | [PH]
6.0-7.5 (50g/l, H2O, 20℃) | [PH Range]
9 | [Stability:]
Stable. Incompatible with strong oxidizing agents. | [biological source]
synthetic (organic) | [Water Solubility ]
1000 g/L (20 ºC) | [Decomposition ]
>=200 °C | [Usage]
Niacinamide USP is used as food additive, for multivitamin preparations and as intermediate for pharmaceuticals and cosmetics. WWW Link | [Detection Methods]
HPLC,NMR | [Merck ]
14,6523 | [BRN ]
383619 | [BCS Class]
1 | [InChIKey]
DFPAKSUCGFBDDF-UHFFFAOYSA-N | [LogP]
-0.38 at 21℃ | [Uses]
Niacinamide is a nutrient and dietary supplement that is an available form of niacin. Nicotinic acid is pyridine beta-carboxylic acid and nicotinamide, which is another term for niacinamide, is the corresponding amide. It is a powder of good water solubility, having a solubility of 1 g in 1 ml of water. Unlike niacin, it has a bitter taste; the taste is masked in the encapsulated form. Used in fortification of cereals, snack foods, and powdered beverages. | [CAS DataBase Reference]
98-92-0(CAS DataBase Reference) | [NIST Chemistry Reference]
Niacinamide(98-92-0) | [EPA Substance Registry System]
98-92-0(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing .
S37/39:Wear suitable gloves and eye/face protection . | [RIDADR ]
UN1230 - class 3 - PG 2 - Methanol, solution | [WGK Germany ]
1
| [RTECS ]
QS3675000
| [F ]
8 | [Autoignition Temperature]
480 °C | [TSCA ]
Yes | [HS Code ]
29362990 | [Safety Profile]
Moderately toxic by
ingestion, intravenous, intraperitoneal, and
subcutaneous routes. Mutation data
reported. When heated to decomposition it
emits toxic fumes of NOx. | [Hazardous Substances Data]
98-92-0(Hazardous Substances Data) |
| Hazard Information | Back Directory | [General Description]
White powder. | [Reactivity Profile]
An amine and amide. Acts as a weak base in solution. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides. Organic amides/imides react with azo and diazo compounds to generate toxic gases. Flammable gases are formed by the reaction of organic amides/imides with strong reducing agents. Amides are very weak bases (weaker than water). Imides are less basic yet and in fact react with strong bases to form salts. That is, they can react as acids. Mixing amides with dehydrating agents such as P2O5 or SOCl2 generates the corresponding nitrile. The combustion of these compounds generates mixed oxides of nitrogen (NOx). | [Air & Water Reactions]
Water soluble. | [Potential Exposure]
Used as a dietary supplement and
food additive. | [First aid]
If this chemical gets into the eyes, remove any
contact lenses at once and irrigate immediately for at least
15 minutes, occasionally lifting upper and lower lids. Seek
medical attention immediately. If this chemical contacts the
skin, remove contaminated clothing and wash immediately
with soap and water. Seek medical attention immediately.
If this chemical has been inhaled, remove from exposure,
begin rescue breathing (using universal precautions, including
resuscitation mask) if breathing has stopped and CPR if
heart action has stopped. Transfer promptly to a medical
facility. When this chemical has been swallowed, get medical
attention. Give large quantities of water and induce
vomiting. Do not make an unconscious person vomit. | [Incompatibilities]
Combustible solid; dust may form explosive
mixture with air. Amides are incompatible with oxidizers
(chlorates, nitrates, peroxides, permanganates,
perchlorates, chlorine, bromine, fluorine, etc.); contact may
cause fires or explosions. Keep away from alkaline materials,
strong bases, strong acids, oxoacids, epoxides. | [Originator]
Niacinamide,Twinlab | [Definition]
ChEBI: A pyridinecarboxamide that is pyridine in which the hydrogen at position 3 is replaced by a carboxamide group. | [Manufacturing Process]
Gaseous ammonia was passed into nicotinic acid at a temperature between
200-235°C until the conversion to nicotinamide was 85%. The reaction
mixture was colored light brown. The reaction mass was cooled and grounds
to a fine powder. Fifty grams of this crude nicotinamide were boiled with 500
ml of anhydrous ethyl acetate until a dark solution was. obtained. A little solid remained in suspension. Gaseous ammonia was passed in below the surface
of the ethyl acetate at a temperature between 60-70°C. After a short time
ammonium nicotinate started to precipitate out of solution as a brown solid.
Sufficient gaseous ammonia, was passed into the ethyl acetate solution to
insure complete precipitation of the nicotinic acids as ammonium nicotinate.
The solution was filtered at about 60-70°C. The filter cake consisted of
ammonium nicotinate, which, upon drying, weighed 12.4 grams. The filtrate
was stirred arid boiled for 20 minutes with one-half gram of activated carbon
and two grams of activated adsorbent clay. The mixture was filtered hot. The
filtrate was boiled twenty minutes with one-half gram of activated carbon and
two grams of activated adsorbent clay and then filtered hot. The carbon and
clay treatment was repeated once more. The final filtrate was cooled slowly
with stirring to room temperature to precipitate white crystalline nieocinamide
which, upon drying, weighed 26.7 grams and had a melting point of 129.5°C,
and was over 99 percent pure. The mother liquor from the above filtration
was boiled down to one-third of its volume and cooled to room temperature. A
second crop of nicotinamide of three grams was obtained. | [Therapeutic Function]
Enzyme cofactor vitamin | [benefits]
Niacinamide is a multipurpose skin care ingredient. It helps build keratin, a protein that maintains skin health.
1. Niacinamide may enhance the function of skin’s lipid barrier (a layer of water and oil that protects your skin). This helps lock moisture in and keep pollutants or other potential irritants out.
2. Niacinamide has been shown to ease inflammation, which can help calm redness due to conditions like acne, rosacea and eczema. It can also soothe irritation caused by strong exfoliants like retinol or glycolic acid.
3. Niacinamide may help minimize pore's appearance by helping keep your skin smooth and clear. It also may help regulate the amount of oil your glands produce, which can prevent breakouts and clogged pores.
4. Oral nicotinamide supplements may help prevent new skin cancer or precancerous spots from developing in some people.
5. Some research suggests skin care formulas with 5% niacinamide can also help lighten dark spots.
| [Synthesis Reference(s)]
Journal of the American Chemical Society, 76, p. 5774, 1954 DOI: 10.1021/ja01651a043
Tetrahedron Letters, 36, p. 8657, 1995 DOI: 10.1016/0040-4039(95)01785-G | [Flammability and Explosibility]
Nonflammable | [Biochem/physiol Actions]
Nicotinamide is an amide derivative of vitamin B3 and a PARP inhibitor | [Clinical Use]
Niacin is used in the treatment of niacin deficiency, which is referred to as pellagra (from the Italian, pelle for “skin” and agra for “dry”). The major systems affected are the gastrointestinal tract (diarrhea, enteritis and stomatitis), the skin (dermatitis), and the CNS (generalized neurological deficits including dementia). Pellagra has become a rare condition in the United States and other countries that require or encourage enrichment of wheat flour or fortification of cereals with niacin. Because the nucleotide form can be synthesized in vivo from tryptophan, pellagra is most often seen in areas where the diet is deficient in both niacin and tryptophan. Typically, maize (corn)-based diets meet this criteria. Niacin deficiency can also result from diarrhea, cirrhosis, alcoholism, or Hartnup disease. It is interesting that niacin deficiency can also, rarely, result from vitamin B6 deficiency (see Vitamin B6 section). Niacin, but not niacinamide, is also one of the few vitamins that are useful in the treatment of diseases unrelated to deficiencies. | [Metabolism]
Nicotinamide is ingested in food as part of pyridine nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP) in plant and animal tissues. After the co?enzymes have separated, nicotinamide is absorbed almost completely in the small intestine. After absorption, nicotinamide is stored as NAD in the liver and excretion occurs via kidneys. Tryptophan is converted to nicotinamide through kynurenine?anthranilate pathway in the liver. Tryptophan can thus satisfy the requirement for dietary nicotinic acid. | [storage]
4°C, protect from light | [Purification Methods]
Crystallise niacin from *benzene. It has solubility in g/ml: H2O (1), EtOH (0.7) and glycerol (0.1). [Methods in Enzymology 66 23 1980, UV: Armarego Physical Methods in Heterocyclic Chemistry (Ed Katritzky, Academic Press) Vol III 83 1971, Beilstein 22 III/IV 389, 22/2 V 80.] | [References]
Zapata-Pérez et al. (2021), NAD+ homeostasis in human health and disease; EMBO Mol. Med., 13 e13943
Guan et al. (2014), Mechanism of inhibition of the human sirtuin enzyme SIRT3 by nicotinamide: computational and experimental studies; PLoS One, 9 e107729
Hwang and Song (2017), Nicotinamide is an inhibitor of SIRT1 in vitro, but can be a stimulator in cells; Mol. Life Sci., 74 3347
Meng et al. (2018), Nicotinamide Promotes Cell Survival and Differentiation as Kinase Inhibitor in Human Pluripotent Stem Cells; Stem Cell Reports, 11 1347
Horwitz et al. (2014), Umbilical cord blood expansion with nicotinamide provides long-term multilineage engraftment; Clin. Invest., 124 3121 |
| Questions And Answer | Back Directory | [description]
Nicotinamide aka Vitamin B3 (niacinamide, nicotinic acid amide) is the pyridine 3 carboxylic acid amide form of niacin. It is a water?soluble vitamin that is not stored in the body. The main source of vitamin in diet is in the form of nicotinamide, nicotinic acid, and tryptophan. The main source of niacin include meat, liver, green leafy vegetables, wheat, oat, palm kernel oil, legumes, yeast, mushrooms, nuts, milk, fish, tea, and coffee.
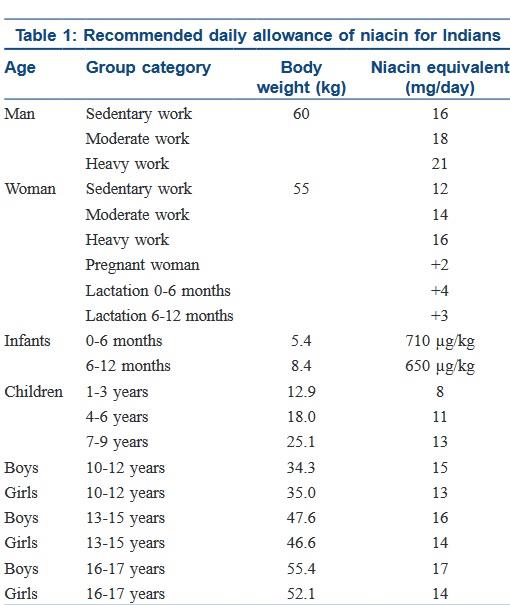
The recommended daily dose of vitamin B3 in niacin equivalent is given in Table 1.
Nicotinamide is a component of coenzyme I (nicotinamide adenine dinucleotide, NADP) and coenzyme II (nicotinamide adenine dinucleotide phosphate, NADP). The nicotinamide part of these two coenzyme structures in human body has reversible hydrogenation and dehydrogenation characteristics, It plays a hydrogen transfer role in biological oxidation, can promote tissue respiration, biological oxidation process and metabolism, and is of great significance to maintain the integrity of normal tissues, especially skin, digestive tract and nervous system. When lacking, pellagra is caused by the influence of cell respiration and metabolism. | [Chemical Properties]
It is white needle crystal or crystalline powder, no smell or odor slightly, slightly bitter taste. The relative density is 1.4, melting point is 131-132 ℃.1 g of the above the product is soluble in 1 ml of water, 1.5 ml ethanol or 10 ml glycerin, insoluble in ether. The pH of 10% aqueous solution is 6.5-7.5. in dry air to light and heat stability, in alkaline or acidic solution, heating generation to nicotinic acid. Rats by oral LD502.5-3.5g/kg ADI value does not make special provisions (ECC, 1990)
| [Uses]
Nicotinamide is a water-soluble B complex vitamin which is naturally present in animal products, whole cereals and legumes. Together with nicotinic acid (niacin), it belongs to vitamin B3 or vitamin PP, and is required as a nutrient to prevent the niacin deficiency disorder pellagra. It functions as a coenzyme or cosubstrate in many biological reduction and oxidation reactions required for energy metabolism in mammalian systems. It is used as a nutritional supplement, therapeutic agent, skin and hair conditioning agent in cosmetics, and a constituent of consumer household solvent and cleaning products and paints. Nicotinamide is approved for use by the FDA as a food additive to enrich corn meal, farina, rice, and macaroni and noodle products. It is also affirmed as GRAS (Generally Recognized as Safe) by the FDA as a direct human food ingredient which includes its use in infant formula. It is approved for use in pesticide products applied to growing crops only as a synergist with a maximum limitation of 0.5% of formulation. | [Toxicity]
LD50 2.5~3.5 g/kg (rats, through the mouth).
GRAS(FDA,§182.5535���,2000).
ADI is no special regulation (EEC, 1990). | [Synthesis]
1.β-methyl pyridine is oxidized to nicotinic acid by air, and the latter is produced by the action of ammonium hydroxide, and then heating and dehydration.
2.Nicotinic acid, boric acid and ammonia into reaction pot, stirring at the condition of ammonia gas, heating dissolution; then distilled ammonia recovery, to 120℃ after the immigration dewatering pot continues to enrich; when the temperature reached 145 ℃, start adding liquid ammonia, and in 185 to 190℃ to ammonia reaction 20~30h. And then cooled to 130℃, diluted with distilled water, activated carbon was added, and in 70~80℃through ammonia decolorization 2h; reaction after filtered, , filtrate in 24 hours after analysis of cold water, fractional crystallization and washing with ethanol, and drying to obtain the finished product. The yield was 89%.
3. From the nicotinic acid and ammonia react into salt and then dehydrated.
|
|
|