| Identification | More | [Name]
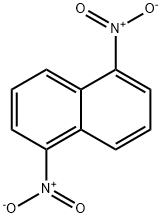
1,5-Dinitronaphthalene | [CAS]
605-71-0 | [Synonyms]
1,5-DINITRONAPHTHALENE
LABOTEST-BB LT00436936
1,5-dinitro-naphthalen
Naphthalene,1,5-dinitro-
1,5-Dinitroaphthalene
1,5-Dinitronaphthalene98%
1,5-Dinitronaphthalene,tech.90%
1,5-DINITRO NAPHTALENE
1,5-Dinitronaphthalene, tech. 90%
1,5-Dinitronaphthalene, 97+% | [EINECS(EC#)]
210-095-2 | [Molecular Formula]
C10H6N2O4 | [MDL Number]
MFCD00003916 | [Molecular Weight]
218.17 | [MOL File]
605-71-0.mol |
| Safety Data | Back Directory | [Hazard Codes ]
Xi,Xn | [Risk Statements ]
R41:Risk of serious damage to eyes.
R43:May cause sensitization by skin contact.
R52/53:Harmful to aquatic organisms, may cause long-term adverse effects in the aquatic environment .
R68:Possible risk of irreversible effects.
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S61:Avoid release to the environment. Refer to special instructions safety data sheet .
S29:Do not empty into drains . | [RIDADR ]
2811 | [WGK Germany ]
3
| [RTECS ]
QJ4551000
| [HazardClass ]
6.1(b) | [PackingGroup ]
III | [Safety Profile]
A suspected
carcinogen. Mutation data reported.
Mxtures with sulfur or sulfuric acid (used in
commercial reactions) may explode if heated
to 120℃. Initiation temperature depends on
the quality of the dinitronaphthalene. When
heated to decomposition it emits toxic
fumes of NOx. See also NITRO COMPOUNDS of AROMATICHYDROCARBONS |
| Hazard Information | Back Directory | [General Description]
Yellowish white needles or light yellow fluffy solid. | [Reactivity Profile]
1,5-DINITRONAPHTHALENE(605-71-0) is incompatible with strong oxidizers and strong bases. Mixtures with sulfur or sulfuric acid may explode if heated to 248° F . | [Air & Water Reactions]
Insoluble in water. | [Fire Hazard]
Flash point data for this chemical are not available; however, 1,5-DINITRONAPHTHALENE is probably combustible. | [Chemical Properties]
Yellow to yellow-green needles or fluffy powder | [Uses]
1,5-Dinitronaphthalene is an intermediate in the production of naphthazarin (5,8- dihydroxy-1,4-naphthoquinone) and 1,5- naphthalenediamine, which is mainly converted to naphthalene 1,5-diisocyanate. As a sensitizing agent for ammonium nitrate explosives the use of mixed isomers is adequate. | [Definition]
ChEBI: A dinitronaphthalene carrying nitro groups at positions 1 and 5. | [Production Methods]
Naphthalene is added slowly to mixed acid (22/58/20) at 40 ℃, and the temperature is raised to 80 ℃ over 4 h to give a mixture of 1,5- and 1,8-dinitronaphthalenes. The isomers are separated by fractional crystallization (e.g., from ethylene dichloride) or, preferably, by solvent extraction. The 1,5-isomer can be extracted with toluene, leaving 99 % pure 1,8- dinitronaphthalene. After evaporation of the toluene, the residue is extracted with a strongly polar solvent (e.g., sulfolane) to leave 99 % pure 1,5-isomer. |
|