| Identification | More | [Name]
Tolazoline | [CAS]
59-98-3 | [Synonyms]
2-BENZYL-2-IMIDAZOLINE
LABOTEST-BB LT00772362
TIMTEC-BB SBB003964
TOLAZOLINE
2-benzyl-2-imidazolin
2-Benzyl-4,5-dihydro-1H-imidazole
2-Benzyl-4,5-imidazoline
2-Benzyl-4,5-imidazoline hydrochloride
2-Benzylimidazoline
2-Imidazoline, 2-benzyl-
4,5-dihydro-2-(phenylmethyl)-1h-imidazol
4,5-Dihydro-2-(phenylmethyl)-1H-imidazole
Artonil
Benzazoline
Benzidazol
Benzolin
Benzylimidazoline
CIBA 3259
ciba3259
Dilatol ASI | [EINECS(EC#)]
200-448-9 | [Molecular Formula]
C10H12N2 | [MDL Number]
MFCD00005182 | [Molecular Weight]
160.22 | [MOL File]
59-98-3.mol |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed .
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S28:After contact with skin, wash immediately with plenty of ... (to be specified by the manufacturer) .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) . | [WGK Germany ]
3
| [RTECS ]
NJ1925000
| [HS Code ]
2933299090 | [Toxicity]
cyt-ham:lng 62,500 mg/L GMCRDC 27,95,81 |
| Hazard Information | Back Directory | [Originator]
Priscoline,Ciba,US,1948 | [Uses]
Reactant for synthesis of imidazoline-containing drug | [Uses]
Tolazoline is used for treating stable forms of pulmonary hypertension in newborns, and in cases
where systemic arterial oxygenation cannot be achieved in the usual manner under careful
observation of professionals. | [Definition]
ChEBI: A member of the class of imidazoles that is 4,5-dihydro-1H-imidazole substituted by a benzyl group. | [Manufacturing Process]
The phenyl-acetiminoether hydrochloride of the formula
from 12 parts of benzylcyanide and ethanol and HCl is mixed with 8 parts of
ethylenediamine hydrate which has been diluted with little alcohol, whereby
the crystals go into solution. The whole is then heated on the water-bath until
the ammonia odor has disappeared, cooled, concentrated caustic potash
solution added, and the separated oil extracted with ether. The solution is
dried with potassium carbonate and potassium hydroxide. After evaporation a
pale oil is left which distills at 147°C under a pressure of 9 mm and which
solidifies in the condenser to a white crystalline mass. The yield amounts to
90% of the theory. The hydrochloride melts at 168° to 170°C. | [Therapeutic Function]
Vasodilator | [General Description]
2-Benzylimidazoline is an active vasodilator. | [Pharmacology]
Tolazoline is a weak, reversible α-adrenoblocker that lowers resistance of peripheral blood
vessels and elevates venous capacity. However, it also exhibits β-adrenomimetic activity,
which consists of the stimulation of cardiac work and is manifest as tachycardia, cholin�ergic activity, which consists of stimulation of the gastrointestinal tract, and histamine-like
activity, which consists of stimulation of gastric secretion. | [Safety Profile]
Poison by ingestion,intraperitoneal, and intravenous routes. Mutation data reported. When heated to decomposition it emits toxicfumes of NOx. | [Synthesis]
Tolazoline, 2-benzyl-2-imidazoline (12.2.7), is synthesized by the heterocy�clation of the ethyl ester of iminophenzylacetic acid with ethylendiamine (12.2.6), which
forms the desired product (12.2.7) [32¨C35]. The structure of tolazoline is strikingly simi�lar to |á-adrenergic agonists, which are antiedema sympathomimetics.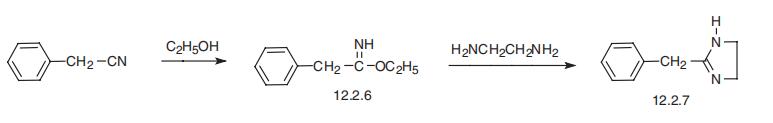
| [storage]
Store at -20°C |
|
| Company Name: |
LGM Pharma
|
| Tel: |
1-(800)-881-8210 |
| Website: |
www.lgmpharma.com |
|