| Identification | More | [Name]
Imidazole | [CAS]
288-32-4 | [Synonyms]
1,3-DIAZA-2,4-CYCLOPENTADIENE
1,3-DIAZOLE
1H-IMIDAZOLE
AKOS BBS-00004265
GLYOXALIN
GLYOXALINE
GLYOXALINE BUFFER
IMIDAZOLE
IMIDAZOLE BUFFER
IMINAZOLE
LABOTEST-BB LTBB001344
N,N'-VINYLENEFORMAMIDINE
1,3-Dia-zole,Miazole
1H-Imidazol
Formamidine, N,N'-vinylene-
glioksal
IMD
Imidazol
Imidazolin
Imutex | [EINECS(EC#)]
206-019-2 | [Molecular Formula]
C3H4N2 | [MDL Number]
MFCD00005183 | [Molecular Weight]
68.08 | [MOL File]
288-32-4.mol |
| Chemical Properties | Back Directory | [Appearance]
White to off white crystals | [Melting point ]
88-91 °C(lit.)
| [Boiling point ]
256 °C(lit.)
| [bulk density]
500-600kg/m3 | [density ]
1.01 g/mL at 20 °C
| [vapor pressure ]
<1 mm Hg ( 20 °C)
| [refractive index ]
1.4801 | [Fp ]
293 °F
| [storage temp. ]
2-8°C
| [solubility ]
H2O: 0.1 M at 20 °C, clear, colorless
| [form ]
crystalline
| [pka]
6.953(at 25℃) | [color ]
white
| [Specific Gravity]
1.03 | [Odor]
Amine like | [PH]
9.5-11.0 (25℃, 50mg/mL in H2O) | [PH Range]
9.5 - 11 | [Stability:]
Stable. Incompatible with acids, strong oxidizing agents. Protect from moisture. | [Water Solubility ]
633 g/L (20 ºC) | [Sensitive ]
Hygroscopic | [λmax]
λ: 260 nm Amax: 0.10
λ: 280 nm Amax: 0.10 | [Merck ]
14,4912 | [BRN ]
103853 | [Dielectric constant]
23.0(Ambient) | [InChIKey]
RAXXELZNTBOGNW-UHFFFAOYSA-N | [LogP]
-0.02 at 25℃ | [CAS DataBase Reference]
288-32-4(CAS DataBase Reference) | [NIST Chemistry Reference]
1H-Imidazole(288-32-4) | [EPA Substance Registry System]
288-32-4(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
C,Xi | [Risk Statements ]
R36/38:Irritating to eyes and skin .
R63:Possible risk of harm to the unborn child.
R34:Causes burns.
R22:Harmful if swallowed.
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S22:Do not breathe dust .
S36:Wear suitable protective clothing .
S27:Take off immediately all contaminated clothing . | [RIDADR ]
UN 2923 8/PG 3
| [WGK Germany ]
1
| [RTECS ]
NI3325000
| [Autoignition Temperature]
480 °C | [TSCA ]
Yes | [HazardClass ]
8 | [PackingGroup ]
III | [HS Code ]
29332900 | [Hazardous Substances Data]
288-32-4(Hazardous Substances Data) | [Toxicity]
LD50 in mice (mg/kg): 610 i.p.; 1880 orally (Nishie) |
| Hazard Information | Back Directory | [Description]
Imidazole (288-32-4) group denotes a heterocyclic organic compound whose molecule contains a five-membered hetero-aromatic ring of two non-adjacent nitrogen atoms, that a carbon atom is placed between two nitrogen atoms. Imidazole is the universally used trivial name for 1,3-azole. Earlier given names were glyoxaline and iminazole. The importance of this aromatic ring system is reflected by its presence in naturally occurring histidine, histamine and the purines, and in several classes of pharmaceuticals. Imidazole can act as a base and as a weak acid. It exists in two tautomeric forms with the hydrogen atom taking position between the two nitrogen atoms.
| [Chemical Properties]
Imidazole(288-32-4) is a heterocyclic compound with a five-membered planar ring. It is amphoteric and highly polar.The pharmacophore of imidazole exists in bioactive compounds including amino acids, plant growth regulators and therapeutic agents. | [History]
Imidazole[288-32-4] was first synthesized in 1858 by Debus from ammo�nia and glyoxal; it was originally named gly�oxalin. The name imidazole was introduced by Hantzsch. Industrial production of imidazole began in the 1950s; a wide range of derivatives is now available in industrial quantities. | [Uses]
Imidazole is used as a buffer in the range of pH 6.2-7.8. It is also an histamine antagonist. It acts as a chelator and forms complexes with various divalent cations. It is used as a corrosion inhibitor on certain transition metals such as copper. Its derivatives, like polybenzimidazole (PBI), act as fire retardants. It finds application in photography and electronics. Imidazole salts are used as ionic liquids and precursors to stable carbenes. Imidazole derivatives like ketoconazole, miconazole and clotrimazole are involved in the treatment of various systemic fungal infections. It is a part of the theophylline molecule, present in tea leaves and coffee beans, which stimulates the central nervous system. | [Application]
Imidazole is a versatile heterocycle used in the preparation of various biologically active compounds such as the amino acid histidine and is present in many antifungal medication. It is also used ext ensively as a corrosion inhibitor on transition metals such as copper.It is used in organic synthesis and as an antiirradiationagent.
Imidazole has been used:
in the lysis, wash and elution buffer for the purification of histidine tagged Sonic Hedgehog(shh-N) protein.
in elution buffer in stepwise gradient for the purification of histidine tagged aldo keto reductases using nickel affinity chromatography.
as a component of homogenization buffer for the purification of phagosomal compartments from dendritic cell. | [Definition]
ChEBI: Imidazole(288-32-4) is an imidazole tautomer which has the migrating hydrogen at position 1. It is a conjugate base of an imidazolium cation. It is a conjugate acid of an imidazolide. It is a tautomer of a 4H-imidazole. | [Preparation]
Imidazole is formed by reacting glyoxal with formaldehyde in the presence of ammonium acetate in acetic acid. The driving energy is microwave radiation. More generally, this reaction is used to produce substituted imidazoles.
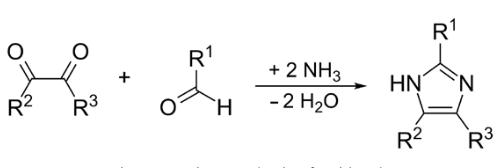
Although there had been discoveries of various derivatives of imidazole in 1840, it was first reported in 1858. The synthesis process of imidazole follows the reaction between formaldehyde in ammonia and glyoxal. This process gives low yield of imidazole but it is still used to form imidazole with C-substitution (Wolkenberg et al., 2004). | [General Description]
Imidazole is a heterocyclic compound with a five-membered planar ring. It is amphoteric and highly polar. The pharmacophore of imidazole exists in bioactive compounds including amino acids, plant growth regulators and therapeutic agents. | [Health Hazard]
It is less toxic relative to pyrrole and otherfive-membered heterocyclic compounds ofnitrogen. Intraperitoneal administration ofimidazole caused somnolence, muscle contractions,and convulsions in mice. Theoral LD50 value in mice is in the range900 mg/kg. | [Fire Hazard]
Noncombustible solid. | [Flammability and Explosibility]
Nonflammable | [Biochem/physiol Actions]
Imidazole derivatives have antibacterial, antifungal and anticancer functionality. It interacts with DNA and also binds to protein and stops cell division. It also acts as a microtubule destabilizing agents and inhibits topoisomerase and Cytochrome P450 Family 26 Subfamily A Member 1 (CYP26A1) enzymes. Imidazole based anticancer drug find applications in cancer chemotherapy. It is used as buffer component for purification of the histidine tagged recombinant proteins in immobilized metal-affinity chromatography (IMAC). | [Mechanism of action]
N-substitution of imidazoles has created a family of drugs, called triazoles, that have the same mechanism of action as imidazoles, a similar or broader spectrum of activity, and less effect on human sterol synthesis. Both imidazoles and triazoles inhibit C-14α demethylation of lanosterol in fungi by binding to one of the cytochrome P-450 enzymes, which leads to the accumulation of C-14α methylsterols and reduced concentrations of ergosterol, a sterol essential for a normal fungal cytoplasmic membrane. Inhibition of cytochrome P-450 also decreases the synthesis of testosterone and glucocorticoids in mammals, an effect seen clinically with ketoconazole but not with later azoles[1].
| [Purification Methods]
Crystallise imidazole from *benzene, CCl4, CH2Cl2, EtOH, pet ether, acetone/pet ether and distilled de-ionized water. Dry it at 40o under vacuum over P2O5. Distil it at low pressure. It is also purified by sublimation or by zone melting. [Snyder et al. Org Synth Coll Vol III 471 1955, Bredereck et al. Chem Ber 97 827 1964, Caswell & Spiro J Am Chem Soc 108 6470 1986.] 15N-imidazole crystallises from *benzene [Scholes et al. J Am Chem Soc 108 1660 1986]. [Beilstein 23 II 34, 23 III/IV 564, 23/4 V 191.] | [References]
[1] Rex, J. and D. Stevens. “39 – Drugs Active against Fungi, Pneumocystis, and Microsporidia.” Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases 23 1 (2015): 479-494. |
|
|