| Identification | Back Directory | [Name]
(R)-(-)-5,5'-Bis[di(3,5-di-t-butyl-4-methoxyphenyl)phosphino]-4,4'-bi-1,3-benzodioxole,min.98%(R)-DTBM-SEGPHOS | [CAS]
566940-03-2 | [Synonyms]
DTBM-SEGPHOS
(R)-DTBM-SEGPHOS
(R)-DTBM-SEGPHOS(R)
(R)-(-)-DTBM-SEGPHOS(regR)
(R)-(-)-5,5'-Bis[di(3,5-di-t-butyl-4-Methoxyphenyl)phosphino]-4,4'-bi-1,3-benzodioxole
(R)-()-5,5′-Bis[di(3,5-di-tert-butyl-4-methoxyphenyl)phosphino]-4,4′-bi-1,3-benzodioxole
[(4R)-(4,4′-bi-1,3-benzodioxole)-5,5′-diyl]bis[bis(3,5-di-tert-butyl-4-methoxyphenyl)phosphine]
(R)-(-)-5,5'-Bis[di(3,5-di-t-butyl-4-methoxyphenyl)phosphino]-4,4'-bi-1,3-benzodioxole, min. 98%
(4R)-[4,4'-Bi-1,3-benzodioxole]-5,5'-diylbis[bis[3,5-bis(1,1-dimethylethyl)-4-methoxyphenyl]phosphine
(R)-(-)-5,5'-Bis[di(3,5-di-t-butyl-4-methoxyphenyl)phosphino]-4,4'-bi-1,3-benzodioxole,min.98%(R)-DTBM-SEGPHOS
(R)-(-)-5,5'-Bis[di(3,5-di-t-butyl-4-methoxyphenyl)phosphino]-4,4'-bi-1,3-benzodioxole, min. 98% (R)-(-)-DTBM-SEGPHOS(R)
[(4R)-(4,4μ-bi-1,3-benzodioxole)-5,5μ-diyl]bis[bis(3,5-di-tert-butyl-4-methoxyphenyl)phosphine], (R)-(-)-5,5μ-Bis[di(3,5-di-tert-butyl-4-methoxyphenyl)phosphino]-4,4μ-bi-1,3-benzodioxole | [Molecular Formula]
C74H100O8P2 | [MDL Number]
MFCD09753003 | [MOL File]
566940-03-2.mol | [Molecular Weight]
1179.55 |
| Chemical Properties | Back Directory | [Melting point ]
126-128°C | [Boiling point ]
987.3±65.0 °C(Predicted) | [form ]
Powder | [color ]
off-white | [InChIKey]
ZNORAFJUESSLTM-UHFFFAOYSA-N |
| Questions And Answer | Back Directory | [Reaction]
- Rhodium catalyzed chemo-, regio, and entantioselective [2 + 2 + 2] cycloaddition of alkynes with isocyanates.
- With copper, enantioselective cross Aldol-type reaction of acetonitrile.
- With copper, enantioselective vinylsilane alkenylation of aldehydes.
- Gold carbene mediated stereoselective cyclopropanation of propargyl esters.
- With copper, enantioselective 1,2-reduction of ketones, and 1,4-reduction of a α,β-usaturated esters.
- With copper, catalytic enantioselective Mannich-type reaction.
- Enantioselective fluorination of b β-keto esters, tert-butoxycarbonyl lactones and lactmes with Sodeoka's Pd-aqua complex and a fluorinating reagent.
- Rh-catalyzed intramolecular olefin or carbonyl hydroacylation.
- Pd-catalyzed γ-arylation of β,γ-unsaturated ketones.
- Involved in numerous conjugate alkynylation, and ring-opening alkynylation of azabenzonorbornadienes.
- Involved in asymmetric hydroamination of bicyclic alkenes/dienes,13a diamination of conjugated dienes,13b and hydroalkoxylation/hydrosulfenylation of allenes.
The actions in the following figures are corresponding to the above ones in sequence.
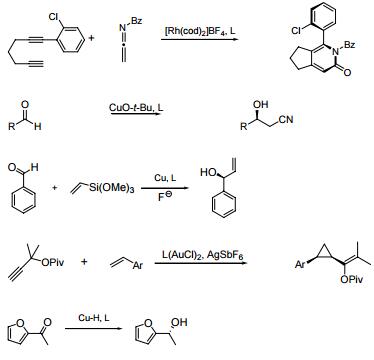


|
| Hazard Information | Back Directory | [Uses]
Catalyst involved in:
- [3,3]-Sigmatropic rearrangements using cyclopropane probes
- Asymmetric intramolecular hydroacylation of ketoaldehydes
Reactant involved in:
- The synthesis of gold-diphosphine complexes for use as catalysts
- Cycloaddition of allenenes to yield alkylidenecyclobutanes
|
|
| Company Name: |
LaaJoo Gold
|
| Tel: |
021-60702684 18516024827 |
| Website: |
http://www.is0513.com/ShowSupplierProductsList20079/0.htm |
| Company Name: |
Energy Chemical
|
| Tel: |
021-021-58432009 400-005-6266 |
| Website: |
http://www.energy-chemical.com |
|