| Identification | Back Directory | [Name]
R(+)-2 2'-BIS-(DIPHENYLPHOSPHINO)-6 6'-& | [CAS]
133545-16-1 | [Synonyms]
SL-A101-1
97% (R)-MeO-BIPHEP
(R)-(+)-MeO-BIPHEP
R(+)-2 2'-BIS-(DIPHENYLPHOSPHINO)-6 6'-&
(R)-(+)-(6,6'-Dimethoxybiphenyl-2,2'-diyl)bis(diphenylphosphine)
(S)-(6,6,-dimethoxy-[1,1,-biphenyl]-2,2,-diyl)bis(diphenylphosphine)
(R)-(+)-2,2'-Bis(diphenylphosphino)-6,6'-diMethoxy-1,1'-biphenyl,(R)-MeO-BIPHEP
(R)-(+)-2,2'-Bis(diphenylphosphino)-6,6'-dimethoxy-1,1'-biphenyl,97% (R)-MeO-BIPHEP
(R)-(+)-2,2'-Bis(diphenylphosphino)-6,6'-dimethoxy-1,1'-biphenyl,min.97%(R)-MeO-BIPHEP
(R)-(+)-(6,6'-Dimethoxybiphenyl-2,2'-diyl)bis(diphenylphosphine) >=97%, optical purity ee: >=99%
(R)-(+)-MeO-BIPHEP, SL-A101-1, (R)-(+)-2,2μ-Bis(diphenylphosphino)-6,6μ-dimethoxy-1,1μ-biphenyl | [Molecular Formula]
C38H32O2P2 | [MDL Number]
MFCD03095430 | [MOL File]
133545-16-1.mol | [Molecular Weight]
582.607 |
| Chemical Properties | Back Directory | [Melting point ]
213-216 °C
| [alpha ]
101 º (C=0.3 IN TOLUENE) | [Boiling point ]
651.1±55.0 °C(Predicted) | [storage temp. ]
under inert gas (nitrogen or Argon) at 2-8°C | [form ]
crystal | [color ]
off-white |
| Hazard Information | Back Directory | [Chemical Properties]
White powder | [Uses]
| [General Description]
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product has been enhanced for catalytic efficiency. Click here for more information. |
| Questions and Answers (Q&A) | Back Directory | [Reactions]
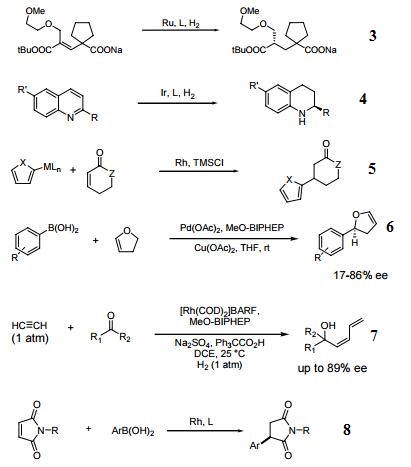
In many respects the catalytic profile of the MeOBIPHEP ligands is similar to that of other atropisomeric diphosphines such as binap and its many analogs. The nature of the PR2 group strongly influences the catalytic performance of the metal complexes. The rhodium and ruthenium MeO-BIPHEP catalysts are highly effective for the hydrogenation of various C=O, C=C and C=N bonds and several synthetically useful C-C coupling reactions.
Ru and Ir catalyzed dynamic kinetic resolution for the synthesis of hydroxy, amino acid derivatives.
- 1.Ru-catalyzed asymmetric hydrogenation of ketones and alkenes.
- Ir catalyzed enantioselective hydrogenation of heteroaromatic compounds.
- Conjugate addition using 2-heteroaryl titanates and zinc reagents.
- Enantio- and regioselective heck-type reaction of aryl boronic acids with 2,3-dihydrofuran
- Rhodium-catalyzed carbonyl Z-dienylation.
- Rhodium-catalyzed asymmetric 1,4 addition of arylboronic acids to maleimides and enones.
|
|
|