| Identification | More | [Name]
6-Maleimidocaproic acid | [CAS]
55750-53-3 | [Synonyms]
6-MALEIMIDOCAPROIC ACID
6-MALEIMIDOHEXANOIC ACID
EMCA
EPSILON-MALEIMIDOCAPROIC ACID
MCA
N-(5-CARBOXYPENTYL)MALEIMIDE
N-EPSILON-MALEIMIDOCAPROIC ACID
N-MALEOYL-6-AMINO-CAPROIC ACID
6-MALEIMIDOCAPROIC ACID, 98+%
6-Maleinimidocaproicacid
6-(2,5-Dioxo-2H-pyrrol-1(5H)-yl)hexanoic acid
6-MALEIMIDOHEXANOIC ACID DIHYDRATE
6-(2,5-dioxo-2,5-dihydro-1H-pyrrol-1-yl)hexanoic acid
6-Maleimidocaproic acid, N-(5-Carboxypentyl)maleimide, N-Maleoyl-6-aminocaproic acid | [EINECS(EC#)]
611-311-1 | [Molecular Formula]
C10H13NO4 | [MDL Number]
MFCD00043140 | [Molecular Weight]
211.21 | [MOL File]
55750-53-3.mol |
| Chemical Properties | Back Directory | [Melting point ]
86-91 °C
| [Boiling point ]
407.3±28.0 °C(Predicted) | [density ]
1.285±0.06 g/cm3(Predicted) | [storage temp. ]
2-8°C
| [solubility ]
DMSO (Slightly), Ethyl Acetate (Slightly) | [form ]
Powder | [pka]
4.74±0.10(Predicted) | [color ]
White to pale yellow | [Water Solubility ]
Soluble in water, methanol, ethanol, dimethyl sulfoxide. Insoluble in ether. | [Sensitive ]
Moisture Sensitive | [Usage]
A sulfhydryl reactive heterobifunctional crosslinking reagent. Widely used probe for introducing maleimides groups into biomolecules. A probe for thiol groups in proteins.
Spacer Arm: 9.4 Angstrom | [BRN ]
1532405 | [InChIKey]
WOJKKJKETHYEAC-UHFFFAOYSA-N | [CAS DataBase Reference]
55750-53-3(CAS DataBase Reference) | [EPA Substance Registry System]
55750-53-3(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing . | [WGK Germany ]
3
| [F ]
10-21 | [HS Code ]
29251900 |
| Hazard Information | Back Directory | [Description]
6-Maleimidocaproic acid contains a maleimide group and a terminal carboxylic acid. The terminal carboxylic acid can react with primary amine groups in the presence of activators (e.g. EDC, or HATU) to form a stable amide bond. The maleimide group will react with a thiol group to form a covalent bond, enabling the connection of biomolecule with a thiol. | [Chemical Properties]
White Solid | [Uses]
A sulfhydryl reactive heterobifunctional crosslinking reagent, 6-Maleimidocaproic acid can be widely used probe for introducing maleimides groups into biomolecules. A probe for thiol groups in proteins. Spacer Arm: 9.4 Angstroms
| [Application]
6-Maleimidocaproic acid is a sulfhydryl reactive heterobifunctional crosslinking reagent. It can be widely used probe for introducing maleimides groups into biomolecules. A probe for thiol groups in proteins. Spacer Arm: 9.4 Angstroms. Probe for thiol groups (SH-groups) in membrane proteins. | [Definition]
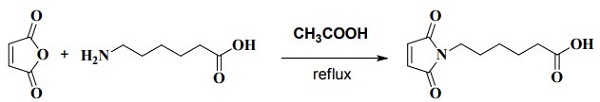
ChEBI: 6-Maleimidocaproic acid is a medium-chain fatty acid. It contains a maleimide group and a terminal carboxylic acid. The terminal carboxylic acid can react with primary amine groups in the presence of activators (e.g. EDC, or HATU) to form a stable amide bond. The maleimide group will react with a thiol group to form a covalent bond, enabling the connection of biomolecule with a thiol. | [Synthesis]
Maleic anhydride (29.4 g, 0.3 mol) and 6-aminocaproic acid (39.35 g, 0.3 mol) were refluxed in
glacial acetic acid (900 mL) for 16 h. Acetic anhydride (30.6 g, 0.3 mol) was added dropwise over
a period of 2 hand reflux was continued for 1 h. The acetic acid was removed under vacuum at 70
°C to yield yellow syrup which solidified. The material was chromatographed over silica using
dichloromethane-methanol-acetic acid (100:5:1) affording a crystalline solid (6-Maleimidocaproic acid).
|
|
|