| Identification | More | [Name]
ZINC TRIFLUOROMETHANESULFONATE | [CAS]
54010-75-2 | [Synonyms]
TRIFLUOROMETHANESULFONIC ACID ZINC SALT
ZINC TRIFLATE
ZINC TRIFLUOROMETHANESULFONATE
ZINC TRIFLUOROMETHANESULPHONATE
trifluoro-methanesulfonicacizincsalt
Zinctrifluoromethanesulfonate,min.98%(Zinctriflate)
Methanesulfonic acid, trifluoro-, zinc salt
trifluoromethanesulfonic acid zinc(ii) salt
zinc(ii) triflate
Zinc trifluoromethanesulphonate 98%
Zinctrifluoromethanesulphonate98%
ZINC TRIFLUOROMETHANESULFONATE (ZINC TRIFLATE)
Trifluoromethanesulfonic acid zinc salt, Zinc triflate
Bis(trifluoromethanesulfonic acid)zinc salt
Bis(trifluoromethylsulfonyloxy) zinc
Zinc bis(trifluoromethanesulfonate) | [EINECS(EC#)]
258-922-6 | [Molecular Formula]
C2F6O6S2Zn | [MDL Number]
MFCD00013229 | [Molecular Weight]
363.53 | [MOL File]
54010-75-2.mol |
| Chemical Properties | Back Directory | [Appearance]
white to light-grey powder | [Melting point ]
≥300 °C(lit.)
| [storage temp. ]
Refrigerator | [form ]
Powder | [color ]
White to light-gray | [Water Solubility ]
Soluble in water and acetonitrile. Slightly soluble in methanol. Insoluble in dichloromethane. | [Sensitive ]
Hygroscopic | [BRN ]
4028195 | [Stability:]
hygroscopic | [InChI]
InChI=1S/2CHF3O3S.Zn/c2*2-1(3,4)8(5,6)7;/h2*(H,5,6,7);/q;;+2/p-2 | [InChIKey]
CITILBVTAYEWKR-UHFFFAOYSA-L | [SMILES]
C(F)(F)(F)S(=O)(=O)[O-].C(F)(F)(F)S([O-])(=O)=O.[Zn+2] | [CAS DataBase Reference]
54010-75-2(CAS DataBase Reference) | [Storage Precautions]
Store under nitrogen | [EPA Substance Registry System]
Methanesulfonic acid, trifluoro-, zinc salt (54010-75-2) |
| Safety Data | Back Directory | [Hazard Codes ]
C | [Risk Statements ]
R34:Causes burns. | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) . | [RIDADR ]
UN 3261 8/PG 2
| [WGK Germany ]
3
| [F ]
3-10 | [Hazard Note ]
Irritant/Hygroscopic | [TSCA ]
Yes | [HazardClass ]
8 | [PackingGroup ]
II | [HS Code ]
29049090 |
| Hazard Information | Back Directory | [Chemical Properties]
white to light-grey powder | [Uses]
Zinc trifluoromethanesulfonate acts as a catalyst for the preparation of dithioketals. It is used as a Lewis acid catalyst in silylation reactions. It is also used as a catalyst for greener amine synthesis by reductive amination with hydrogen gas. | [General Description]
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product has been enhanced for catalytic efficiency. Click here for more information. | [reaction suitability]
core: zinc
reagent type: catalyst | [Synthesis]
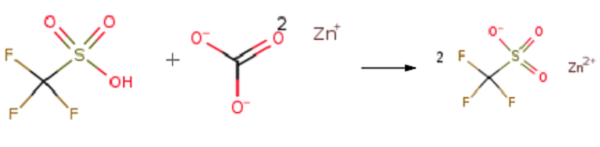
Triflic acid (0.056mol) was added dropwise to a suspension of zinc carbonate (0.02 mol)in dry methanol (20 ml) at room temperature. During the addition, CO2 was evolved. The mixture was stirred at 25°C for 20 minutes and refluxed for 2 h. The clear solution was cooled to 25°C and concentrated under reduced pressure. The resulting white powder was dried at 125°C for 2 h to afford Zinc trifluoromethanesulfonate (Zn(OTf)2 )(98% yield).
| [Purification Methods]
It should be dried at 125o for 2hours at 3mm before use. It is soluble in CH2Cl2 but insoluble in pet ether. [Corey & Shimoji Tetrahedron Lett 24 169 1983.] |
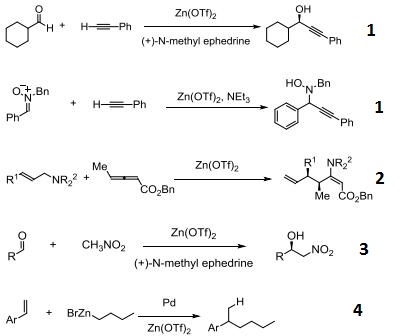
| Questions And Answer | Back Directory | [Reaction]
- Catalyst for the addition of acetylenes to carbonyls and nitrones.
- Claisen rearrangement.
- Catalyst for the enantioselective Henry and Aza-Henry reactions.
- Pd-catalyzed hydroalkylation of styrenes with zinc reagents.
|
|
|