| Identification | More | [Name]
Apigenin | [CAS]
520-36-5 | [Synonyms]
4',5,7-TRIHYDROXYFLAVONE
4,5,7-TRIHYDROXYFLAVONE
4',5,7-TRIHYROXYFLAVONE
5,7,4'-TRIHYDROXYFLAVONE
5,7-DIHYDROXY-2-(4-HYDROXYPHENYL)-4H-1-BENZOPYRAN-4-ONE
7,4',5-TRIHYDROXYFLAVONE
APIGENIN
CHAMOMILE
2-(p-hydroxyphenyl)-5,7-dihydroxychromone
4’,5,7-trihydroxyflavaone
4’,5,7-trihydroxy-flavon
5,7-dihydroxy-2-(4-hydroxyphenyl)-4h-1-benzopyran-4-on
apigenine
apigenol
c.i.naturalyellow1
5,7-dihydroxy-2-(4-hydroxyphenyl)-4-benzopyrone
4,5,7-Trihydroxyflavone (apigenin)
APIGENIN CRYSTALLINE
APIGENIN FROM PARSLEY
Matricaria chamomilla flower | [EINECS(EC#)]
208-292-3 | [Molecular Formula]
C15H10O5 | [MDL Number]
MFCD00006831 | [Molecular Weight]
270.24 | [MOL File]
520-36-5.mol |
| Chemical Properties | Back Directory | [Appearance]
Pale Yellow Crystalline Solid | [Melting point ]
>300 °C (lit.) | [Boiling point ]
333.35°C (rough estimate) | [density ]
1.2319 (rough estimate) | [refractive index ]
1.6000 (estimate) | [storage temp. ]
−20°C
| [solubility ]
DMSO: 27 mg/mL
| [form ]
powder
| [pka]
6.53±0.40(Predicted) | [color ]
yellow
| [biological source]
parsley | [Usage]
Induces the reversion of transformed phenotypes of v-H-ras-transformed NIH 3T3 cells at low concentration (12.5 uM) by inhibiting MAP kinase activity. Also inhibits the proliferation of malignant tumor cells by G2/M arrest and induces morphological | [Merck ]
14,730 | [BRN ]
262620 | [Stability:]
Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 1 month. | [InChI]
InChI=1S/C15H10O5/c16-9-3-1-8(2-4-9)13-7-12(19)15-11(18)5-10(17)6-14(15)20-13/h1-7,16-18H | [InChIKey]
KZNIFHPLKGYRTM-UHFFFAOYSA-N | [SMILES]
C1(C2=CC=C(O)C=C2)OC2=CC(O)=CC(O)=C2C(=O)C=1 | [LogP]
3.020 | [CAS DataBase Reference]
520-36-5(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing . | [WGK Germany ]
3
| [RTECS ]
LK9276000
| [F ]
10 | [HS Code ]
29329900 | [Hazardous Substances Data]
520-36-5(Hazardous Substances Data) |
| Questions And Answer | Back Directory | [Description]
Apigenin(520-36-5) is one of the most widespread flavonoids in plants and formally belongs to the flavone sub-class. Of all the flavonoids, apigenin is one of the most widely distributed in the plant kingdom, and one of the most studied phenolics. Apigenin is present principally as glycosylated in significant amount in vegetables (parsley, celery, onions) fruits (oranges), herbs (chamomile, thyme, oregano, basil), and plant-based beverages (tea, beer, and wine). Plants belonging to the Asteraceae, such as those belonging to Artemisia, Achillea, Matricaria, and Tanacetum genera, are the main sources of this compound.
|
| Hazard Information | Back Directory | [Chemical Properties]
Pale Yellow Crystalline Solid | [Occurrence]
Chamomile is a perennial found in Europe. | [Uses]
antispasmodic, antineoplastic, topoisomerase I inhibitor | [Uses]
Has been used to dye Cr mordanted wool yellow. The color is fast to soap. | [Uses]
Induces the reversion of transformed phenotypes of v-H-ras-transformed NIH 3T3 cells at low concentration (12.5 uM) by inhibiting MAP kinase activity. Also inhibits the proliferation of malignant tum
or cells by G2/M arrest and induces morphological differentiation. Apigenin has also been reported to enhance the gap juntion intracellular communication in liver cells. | [Definition]
ChEBI: Apigenin is a trihydroxyflavone that is flavone substituted by hydroxy groups at positions 4', 5 and 7. It induces autophagy in leukaemia cells. It has a role as a metabolite and an antineoplastic agent. It is a conjugate acid of an apigenin-7-olate. | [Preparation]
4-hydroxybenzaldehyde (1.22 g, 9.97 mmol, 1.0 equiv) was added to asolution of 50% KOH (aq.) (6.72 g, 59.82 mmol, 6.0 equiv) and ethanol (3 mL) andstirred for 10 min. Then compound 9 (2.02g, 9.97 mmol, 1.0 equiv) was added to the reaction mixture and heated to 60 °C and stirred for 4 h. After cooled toroom temperature, the mixture was poured into ice water and acidified withconcentrated hydrochloric acid to pH = 3. Then the suspension was filtrated, washed and the residue was dried toafford Apigenin(2.43 g, 90%) as a red solid. 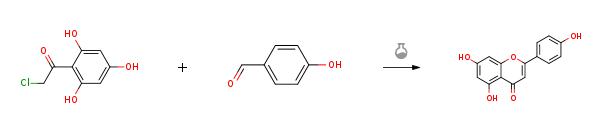
| [benefits]
Apigenin has various beneficial health effects such as antioxidant, anti-inflammatory, and chemoprevention. Apigenin can downregulate the expression of IL-1β and TNF-α in LPS-stimulated mouse macrophages and human monocytes.
preclinical studies have suggested that apigenin may improve outcomes in multiple health states, including anxiety, brain function, oxidative stress, inflammation, and hormonal regulation (testosterone, estrogen, and cortisol | [General Description]
The herb known as chamomile is derived from the plants Matricaria chamomilla (German, Hungarian, or genuine chamomile) and Anthemis nobilis (English, Roman, or common chamomile). Plants from the two genera have similar activities. The medicinal components are obtained from the flowering tops. The flowers are dried and used for chamomile teas and extracts. Chamomile has been used medicinally for at least 2,000 years. The Romans used the herb for its medicinal properties, which they knew were antispasmodic and sedative.
The herb also has a long history in the treatment of digestive and rheumatic disorders. The activity of chamomile is found in a light blue essential oil that composes only 0.5% of the flower. The blue color is caused by chamazulene, 7-ethyl-1,4-dimethylazulene . This compound is actually a byproduct of processing the herb. The major component of the oil is the sesquiterpene (-)-α-bisabolol. Also present are apigenin, angelic acid, tiglic acid, the terpene precursors (farnesol, nerolidol, and germacranolide) coumarin, scopoletin-7-glucoside, umbelliferone, and herniarin. Much of the effect of chamomile is caused by bisabolol . Bisabolol is a highly active anti-inflammatory agent in various rodent inflammation and arthritis tests. | [Biological Activity]
Protein kinase inhibitor. Suppresses tumor-promoting effects of TPA and exhibits antiproliferative activity in human breast cancer cells (IC 50 values are 59.44 and 31.15 μ M at 24 and 72 hrs respectively). Activates both the intrinsic and extrinsic apoptotic pathways and displays anti-inflammatory, hypotensive, antispasmodic and antioxidant properties in vivo . | [Biochem/physiol Actions]
A plant flavonoid that has been found to inhibit cell proliferation by arresting the cell cycle at the G2/M phase. Inhibition of growth through cell cycle arrest and induction of apoptosis appear to be related to induction of p53. Inhibits PMA-mediated tumor promotion by inhibiting protein kinase C and the resulting suppression of oncogene expression. It has also been reported to inhibit topoisomerase I-catalyzed DNA re-ligation and enhance gap junctional intercellular communication. | [Mechanism of action]
Apigenin exerts anxiolytic effects at high doses by inhibiting NMDA receptors; it also has affinity to GABA-A receptors. Apigenin also exerts potent antioxidant activities by scavenging free radicals and upregulating glutathione levels; it also exerts anti-inflammatory effects. Apigenin is most studied for its potential anti-cancer properties. | [Anticancer Research]
It is a flavone compound found in many fruits and vegetables and abundant in chamomiletea, parsley, celeriac, and celery. It induces apoptosis by targeting leptin/leptin receptor pathway and by targeting caspase-dependent extrinsic pathway aswell as STAT3 signaling pathway in lung adenocarcinoma and BT-474 breast cancercells, respectively (Singh et al. 2016b). It shows antitumor activity against breastcancer MCF-7 cells and colon cancer HCT 116 cells and is a mediator of cancerchemoprevention and an inducer of autophagy. It can be used to treat colon canceras it induces apoptosis in colon cancer cells. It also increase melanogenesis in B16cells by activating the p38 MAPK pathway (Wang et al. 2012). | [Safety]
Apigenin is considered safe when consumed in normal amounts through a diet rich in fruits, vegetables, and herbs. However, supplement doses tend to deliver a significantly higher amount of apigenin than would be generally consumed via dietary means. Higher doses of apigenin can cause stomach discomfort, and people should cease using it immediately and consult medical professionals. At high doses, it can trigger muscle relaxation and sedation[1]. Allergic reactions can also occur as a response to chamomile tea or apigenin.
| [Source]
Apigenin (4′,5,7-trihydroxyflavone) is one of the most widespread flavonoids in plants and formally belongs to the flavone sub-class. Plants belonging to the Asteraceae, such as those belonging to Artemisia, Achillea, Matricaria, and Tanacetum genera, are the main sources of this compound. However, species belonging to other families, such as the Lamiaceae, for instance, Sideritis and Teucrium, or species from the Fabaceae, such as Genista, showed the presence of apigenin in the aglycone form and/or its C- and O-glucosides, glucuronides, O-methyl ethers, and acetylated derivatives. In gymnosperms, apigenin derivatives are mostly present in dimeric forms, with apigenin residues variously coupled, e.g., with C?C linkage as in cupressuflavone and amentoflavone (I-8, II-8″ and I-3′, II-8″, respectively), or C—O linkage (I-4′, II-6″) as in hinokiflavone. | [storage]
Store at -20°C | [Purification Methods]
Crystallise it from aqueous pyridine or aqueous EtOH. It dyes wool yellow when mixed with Cr ions. [Beilstein 18 H 181, 18 I 396, 18 II 178, 18 III/IV 2682, 18/4 V 574.] | [References]
[1] Bahare Salehi, et al. “The Therapeutic Potential of Apigenin.” Int J Mol Sci. 2019 Mar; 20(6): 1305.
|
|
|