| Identification | More | [Name]
5-Isopropyl-2-methylphenol | [CAS]
499-75-2 | [Synonyms]
2-HYDROXY-4-CYMENE
2-HYDROXY-4-ISOPROPYL-1-METHYLBENZENE
2-HYDROXY-P-CYMENE
2-METHYL-5-(1-METHYLETHYL)PHENOL
2-METHYL-5-ISOPROPYLPHENOL
5-ISOPROPYL-2-METHYLPHENOL
5-ISOPROPYL-O-CRESOL
CARVACROL
CYMOPHENOL
FEMA 2245
Hydroxy-p-cymene
ISOTHYMOL
Methyl-5-(1-methylethyl)phenol
P-CYMEN-2-OL
1-Hydroxy-2-methyl-5-isopropylbenzene
2-Hydroxy-1-methyl-4-(1-methylethyl)benzene
2-hydroxy-4-(2-propyl)toluene
2-hydroxy-p-cymen
2-methyl-5-(1-methylethyl)-pheno
2-para-cymenol | [EINECS(EC#)]
207-889-6 | [Molecular Formula]
C10H14O | [MDL Number]
MFCD00002236 | [Molecular Weight]
150.22 | [MOL File]
499-75-2.mol |
| Chemical Properties | Back Directory | [Appearance]
liquid | [Melting point ]
3-4 °C(lit.)
| [Boiling point ]
236-237 °C(lit.)
| [density ]
0.976 g/mL at 20 °C(lit.)
| [vapor pressure ]
3.09-6.664Pa at 25℃ | [FEMA ]
2245 | [refractive index ]
n20/D 1.522(lit.)
| [Fp ]
224 °F
| [storage temp. ]
-20°C | [solubility ]
1.25g/l | [form ]
neat | [pka]
10.38±0.10(Predicted) | [color ]
Colorless to Light orange to Yellow | [Odor]
at 100.00 %. spice woody camphor thymol | [Stability:]
Stable. Combustible. Incompatible with strong bases, strong oxidizing agents. | [biological source]
synthetic | [Odor Type]
spicy | [Water Solubility ]
Insoluble | [JECFA Number]
710 | [Merck ]
1872 | [BRN ]
1860514 | [InChIKey]
RECUKUPTGUEGMW-UHFFFAOYSA-N | [LogP]
3.33-3.49 | [Uses]
Carvacrol is a flavoring agent that is a colorless to pale yellow liquid.
it has a spicy and pungent odor, resembling thymol. it is insoluble in
water and soluble in alcohol and ether. it is a mixture of the isomeric
carvacrols (isopropyl o-creols), and is obtained by chemical synthesis.
it is also an ingredient of savory, a fragrant herb in nature. | [CAS DataBase Reference]
499-75-2(CAS DataBase Reference) | [NIST Chemistry Reference]
Phenol, 2-methyl-5-(1-methylethyl)-(499-75-2) | [EPA Substance Registry System]
499-75-2(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
C | [Risk Statements ]
R22:Harmful if swallowed.
R34:Causes burns. | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) . | [RIDADR ]
UN 3265 8/PG 3 | [WGK Germany ]
3 | [RTECS ]
FI1225000 | [HazardClass ]
8 | [PackingGroup ]
III | [HS Code ]
29071990 | [Safety Profile]
Poison by ingestion,
intravenous, and subcutaneous routes.
Moderately toxic by skin contact. A severe
skin irritant. Combustible liquid. When
heated to decomposition it emits acrid
smoke and irritating fumes. | [Hazardous Substances Data]
499-75-2(Hazardous Substances Data) |
| Raw materials And Preparation Products | Back Directory | [Raw materials]
Eucalyptus Citriodara Oil-->Thymol-->Bicyclo[4.1.0]hept-3-en-2-one, 4,7,7-trimethyl--->Bicyclo[4.1.0]hept-3-en-2-one, 3,7,7-trimethyl--->2,4-Cycloheptadien-1-one, 3,6,6-trimethyl--->Bicyclo[4.1.0]hept-4-en-3-one, 4,7,7-trimethyl--->2,6,6-TRIMETHYL-2,4-CYCLOHEPTADIEN-1-ONE-->5-ISOPROPYL-3-METHYLPHENOL | [Preparation Products]
Acetamide-->2-(5-ISOPROPYL-2-METHYL-PHENOXY)-ETHYLAMINE |
| Questions And Answer | Back Directory | [Artificial flavor]
Carvacrol is the isomeric body of thymol with similar aroma as thymol. Carvacrol naturally exists in thyme oil and other essential oils, having especially high content in the thyme oil produced in Spain.
Carvacrol is mainly used in the preparation of dill, girofle, mint and vanilla flavor to be used for toothpaste, toothpaste powder, oral products, talcum powder, soap and daily industrial products. In medicine, it is used for local anesthetics. In addition, carvacrol is capable of killing bacteria and intestinal parasites and can be used as disinfectants and fungicides. According to the information reported, the acute toxicity data of carvacrol: oral LD50810mg/kg (rat), skin test: LD50> 5g/kg (rabbit).
Thyme plant has its origin including the Northeast, Hebei, and Inner Mongolia, Gansu, Qinghai and Xinjiang and other provinces. Ingredients: whole plant of thyme contains about 0.15~0.5% volatile oil (in oil, the component mainly contains carvacrol, paraffin, thymol), amaroid and tannin. Leaves contain free oleanolic acid, ursolic acid, caffeic acid and so on. Thyme can be not only used in medicine, for spices, but also be as the superior natural health ingredients of the processed foods. Since the 1980s, thyme-like herbal foods have become very popular. Studies have shown that thyme-containing volatile oils, in addition to being used as seasoning and for treatment of illnesses, also have anti-corrosion, antibacterial and other functions. Therefore, the consumers can benefit a lot using it as food raw materials. Because of this, in Europe, natural herbal tea also contains thyme. For example, a kind of herb tea raw material having anti-cough effect and being able to prevent and treat bronchitis has 10% thyme leaves.

Image 1: Thymus plant | [Chemical properties]
Use sulfuric acid to sulfonate the p-isopropyl toluene, generating p-isopropyl toluene-2-sulfonic acid. Then we can obtain carvacrol after alkali melting treatment.
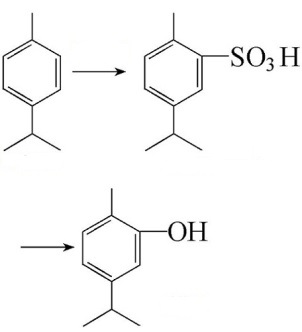
Image 2: Preparation of carvacrol from p-isopropyltoluene
Carvacrol, when co-melted together with potassium hydroxide, can give 2-hydroxy-4-isopropyl benzoic acid while reaction with phosphorus pentachloride can generate 2-chloro-1-methyl-4-isopropyl benzene. In acetic acid, it reaction with 1 mol Bromine can generate 4-bromocarbinol while its reaction with 2 mol bromine can generate 4, 6-dibromo carvacrol; if reacted with bromine and aluminum, we can obtain tetrabromo-o-cresol. It is easily soluble in concentrated sulfuric acid to produce 2-methyl-5-isopropyl phenol-4-sulfonic acid. In aqueous sodium hydroxide solution, it can be reacted with formaldehyde to generate carvacrol phenols alcohol:
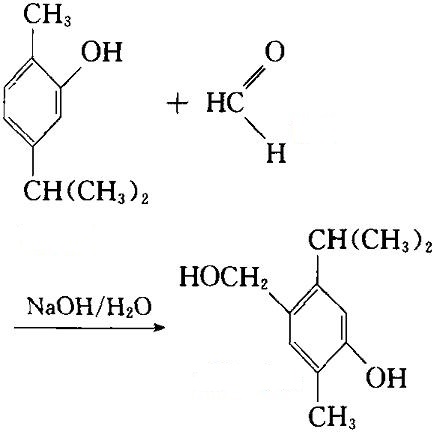
Image 3: The reaction between carvacrol and formaldehyde for generation of carvacrol phenolic alcohol
It exhibits green color when reacted with ferric chloride in ethanol with no significant color in the water. | [Content analysis]
It can be determined by the non-polar column method by GT-10-4. | [Toxicity]
LD orally in rabbits: 100 mg/kg (Kochmann) | [Usage limit]
FEMA (mg/kg): soft drinks 26; cold drinks 34; candy 92; baked goods 120; condiments 37.
Take appropriate as limit (FDA § 172.515, 2000).
Allow to use (GB 2760-1996). | [Standard for Maximum Allowable Amount]
Name of additive
Field allowed to use it as additive
Function of additive
Maximal allowable amount(g/kg)
arvacrol
Food
Food spices
The perfume ingredients used in formulating fragrances shall not exceed the maximum allowable documented amount in GB 2760
| [Chemical properties]
It appears as colorless to pale yellow slightly viscous oil. When placed in air and light, its color becomes darker. It has spicy, cool incense, herbs incense with thymol-like smell. It has a boiling point of 238 ° C, the melting point of 0.5 to 1 ° C and the flash point of 100 ° C. It is soluble ethanol, ethyl ether, propylene glycol and alkali, do not dissolve in water. It is miscible in oil.
The natural products are found in thyme oil (about 70%), oregano oil (about 80%) and mange to oil and so on. | [Application]
Spices; It is mainly used for the preparation of dill, girofle, meat, peppermint, vanilla flavor and so on.
It can be used for the preparation of spices, fungicides and disinfectants. As a spice, it can be used in toothpaste, soap and other daily necessities, but also for food flavors.
It can be used for spices, food additives, feed additives, antioxidants, sanitation fungicide, insect repellent, preservatives, deodorant and pharmaceutical intermediates. | [Preparation]
It can be obtained through treatment of the essential oil containing rich content of natural carvacrol followed by ether extraction or steam distillation to derive it.
It can be derived by the sulfonation of thymol followed by alkali treatment. | [Category]
Pesticides | [Toxic classification]
middle-level poisoning | [Acute toxicity]
Oral-rat LD50: 810 mg/kg; intravenous-mouse LD50: 80 mg/kg
Stimulation Data Skin-Rabbit 500 mg/24 h Severe | [Flammability and Hazardous characteristics]
It is flammable upon heat, fire and strong oxidants with thermal decomposition releasing spicy stimulated smoke | [Storage and transportation characteristics]
Treasury: low temperature, ventilated and dry; avoid fire and high temperature; store it separately from oxidant. | [Extinguishing agent]
fog, carbon dioxide, foam, dry powder |
| Hazard Information | Back Directory | [Occurrence]
Reported found in cumin seed, thyme, oregano, calamus, lovage, myrtle and lemon balm. | [Definition]
ChEBI: A phenol that is a natural monoterpene derivative of cymene. An inhibitor of bacterial growth, it is used as a food additive. Potent activator of the human ion channels transient receptor potential V3 (TRPV3) and A1 (TRPA1). | [Aroma threshold values]
Detection at 2.29 ppm. | [Taste threshold values]
Taste characteristics at 5.0 ppm: spicy, herbal phenolic, medicinal and woody. | [Synthesis Reference(s)]
Tetrahedron Letters, 19, p. 2545, 1978 DOI: 10.1016/S0040-4039(01)94822-1 | [General Description]
Produced and qualified by HWI pharma services GmbH.
Exact content by quantitative NMR can be found on the certificate. | [Flammability and Explosibility]
Notclassified |
|
|