| Identification | Back Directory | [Name]
Vinpocetine | [CAS]
42971-09-5 | [Synonyms]
Tcv-3B
CAVINTON
RGH-4405
AY-27255
Ceractin
(3α,16α)-
VINPOCETIN
VINPOCETINE
Ultra-Vinca
Vinpoeetine
EBURNAMENINE
Vinpocetine EP
VINPOCETINE(RG)
Ceratin:RGH-4405
ethylapovincaminate
Ethyl apovincaminate
Vinpocetine (100 mg)
(3α,16α)-Eburnamenine
Vinpocetine98.0-102.0%
apovincaminated’ethyle
ETHYL APOVINCAMIN-22-OATE
Ethyl (+)-cis-apovincaminate
VINPOCETINE, MM(CRM STANDARD)
Apovincaminic acid ethyl ester
ethyl eburnamenine-14-carboxylate
cis-Apovincaminic acid ethyl ester
(+)-Apovincaminic acid ethyl ester
3A,16A-APOVINCAMINIC ACID ETHYL ESTER
(+)-cis-Apovincaminic acid ethyl ester
EBURNAMENINE-14-CARBOXYLIC ACID ETHYL ESTER
3-alpha,16-alpha-apovincaminicacidethylester
(3α,16α)-Eburnamenine-14-carboxylic acid ethyl
3-alpha,16-alpha-Apovincaminic acid ethyl ester
Ethyl (3alpha,16alpha)-eburnamenine-14-carboxylate
(3α��,16α)-Eburnamemne-14-carboxylic acid ethyl ester
(3A,16A)-EBURNAMENINE-14-CARBOXYLIC ACID ETHYL ESTER
(3α, 16α)-eburnamenine-14-carboxylic acid ethyl ester
ethylester,(3-alpha,16-alpha)-eburnamenine-14-carboxylicaci
(3alpha,16alpha)-Eburnamenine-14-carboxylic acid ethyl ester
Eburnamenine-14-carboxylic acid, ethyl ester, (3a,16a)-(9CI)
Eburnamenine-14-carboxylic acid, ethyl ester, (3alpha,16alpha)-
Eburnamenine-14-carboxylic acid ethyl ester, (3α, 16α)-Eburnamenine-14-carboxylic acid ethyl ester
(41S,13aS)-ethyl 13a-ethyl-2,3,41,5,6,13a-hexahydro-1H-indolo[3,2,1-de]pyrido[3,2,1-ij][1,5]naphthyridine-12-carboxylate
3a,16a-Eburnamenine-14-carboxylic Acid Ethyl Ester, 3a,16a-Apovincaminic Acid Ethyl Ester, Ethyl Apovincamin-22-oate, RGH-4405, Cavinton | [EINECS(EC#)]
256-028-0 | [Molecular Formula]
C22H26N2O2 | [MDL Number]
MFCD00211233 | [MOL File]
42971-09-5.mol | [Molecular Weight]
350.45 |
| Chemical Properties | Back Directory | [Appearance]
White Crystalline Solid | [Melting point ]
147-153 °C dec. | [alpha ]
D20 +114° (c = 1 in pyridine) | [Boiling point ]
484.44°C (rough estimate) | [density ]
1.1260 (rough estimate) | [refractive index ]
1.6500 (estimate) | [storage temp. ]
Store at RT | [solubility ]
DMSO: 5 mg/mL
| [form ]
solid
| [pka]
7.87±0.60(Predicted) | [color ]
white
| [optical activity]
[α]/D 148.5±5.0°, c = 0.1 in chloroform | [λmax]
315nm(EtOH)(lit.) | [Merck ]
14,9991 | [Stability:]
Photosensitive | [InChIKey]
DDNCQMVWWZOMLN-IRLDBZIGSA-N | [LogP]
5.137 (est) | [NIST Chemistry Reference]
Vinpocetine(42971-09-5) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
22 | [Safety Statements ]
36 | [WGK Germany ]
3
| [RTECS ]
JW4792000
| [HS Code ]
29399900 | [Toxicity]
LD50 in mice, rats (mg/kg): 534, 503 orally; 240, 133.8 i.p.; 58.7, 42.6 i.v. (Pálosi, Szporny), also reported as 161.2 mg/kg i.p. in mice (Cholnoky, Dmk) |
| Hazard Information | Back Directory | [Biological Activity]
Phosphodiesterase inhibitor, selective for PDE1 (IC 50 = 21 μ M). Also blocks voltage-gated Na + channels. | [Description]
Vinpocetine (42971-09-5) is a phosphodiesterase PDE1 inhibitor (IC50=21 μM).1?Also blocks voltage-gated Na+?channels, IC50=44.2 μM (potency similar to phenytoin), a mechanism which may contribute to its neuroprotective and anticonvulsant activity.2?It reduces inflammatory IL-1β and TNF-α expression in rat hippocampus.3?Displays beneficial effects in a rat model of cerebral ischemia-reperfusion injury.4?Vinpocetine exerts neuroprotective effects by suppressing microglial inflammation.5 | [Uses]
A calcium/calmodulin-dependent phosphodiesterase 1 (PDE1) inhibitor | [Uses]
A derivative of Vincamine with vasodilating activity. Vasodilator (cerebral). | [Uses]
calcium regulator | [Uses]
Gleevec metabolite, tyrosine kinase inhibitor | [Definition]
ChEBI: Vinpocetine is an alkaloid. It has a role as a geroprotector. | [Biochem/physiol Actions]
Ca2+-calmodulin-dependent phosphodiesterase I (PDE1) inhibitor. | [target]
NF-kB | Beta Amyloid | Caspase | IL Receptor | TNF-α | Akt | JNK | Bcl-2/Bax | ROS | PI3K | IkB | MAPK | IKK | [storage]
Store at -20°C | [References]
1) Hagiwara?et al. (1984),?Effects of vinpocetine on cyclic nucleotide metabolism in vascular smooth muscle; Biochem. Pharmacol.,?33?453
2) Molnar and Erdo (1995),?Vinpocetine is as potent as phenytoin to block voltage-gated Na+ channels in rat cortical neurons; Eur. J. Pharmacol.,?273?303
3) Gomez?et al. (2014),?The anti-seizure drugs vinpocetine and carbamazepine, but not valproic acid, reduced inflammatory IL1β and TNF-α expression in rat hippocampus; J. Neurochem.,?130?770
4) Wang?et al. (2014),?Anti-inflammatory effects of vinpocetine on the functional expression of nuclear factor-kappa B and tumor necrosis factor-alpha in a rat model of cerebral ischemia-reperfusion injury; Neuro. Sci. Lett.,?566?247
5) Zhao?et al.?(2011), TSPO-specific ligand vinpocetine exerts a neuroprotective effect by suppressing microglial inflammation; Glia Biol.,?7?187 |
| Questions And Answer | Back Directory | [Cerebral Vasodilator]
Vinpocetine is a cerebrovascular expansion drug and has a relatively stronger function than Vincamine. It can selectively increase cerebral blood flow, enhance and improve brain oxygen supply, promote metabolism, enhance the capacity of deformation for red blood cells, reduce blood viscosity, inhibit platelet aggregation, improve tissue metabolism. Vinpocetine is mainly used in the treatment of cerebral infarction sequela, cerebral hemorrhage sequelae, cerebral arteriosclerosis, etc. It can also used in the treatment of retinal vascular sclerosis and blood vessel spasm, the elderly deafness, and dizziness.
Vinpocetine is a half synthetic Vincamine derivative, and has the similar effect with Vincamine. It has a stronger expansion function for cerebrovascular selectively. Pharmacological effects are as follows:
①Inhibit the activity of calcium dependency phosphodiesterase, increase the content of cAMP which can relax vascular smooth muscle, then relax vascular smooth muscle, and further increase cerebral blood flow.
②Enhance the capacity of deformation of red blood cells, reduce blood viscosity, inhibit platelet aggregation, and thus improve blood flow and microcirculation.
③Promote the brain tissue to absorb glucose, and promote the transformation of brain monoamine metabolism.
④Inhibit the increase of brain lactic acid during cerebral ischemia, increase the ATP content, inhibit the generation of lipid peroxide in the brain, delay the occurring of spasm caused by cerebral ischemia, have the function of improving cerebral metabolism and protecting brain.
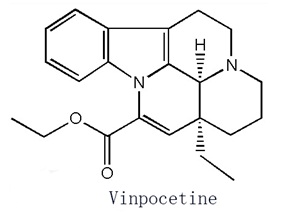
Figure 1 is the structural formula of vinpocetine. | [Pharmacokinetics]
Vinpocetine is of high fat-solubility, easy to be absorbed by the organization, and widely distributed. It can through the blood brain barrier, mainly metabolism to Vinpocetine in the liver, and be excreted by the kidney. | [Medicinal properties and Application]
Vinpocetine is also called Ethyl apovincaminate, Conway, Karan and Vinpocetine. It is a kind of natural medicine extracted from small vinca flower, and belongs to the indole alkaloids. Can be synthetic now. The mechanism of its pharmacological action is to inhibit the activity of calcium dependency phosphodiesterase, increase the content of CGMP which can relax vascular smooth muscle, then relax vascular smooth muscle, and further increase cerebral blood flow; Enhance the capacity of deformation of red blood cells, reduce blood viscosity, inhibit platelet aggregation, and thus improve blood flow and microcirculation; Promote the brain tissue to absorb glucose, and promote the transformation of brain monoamine metabolism; Inhibit the increase of brain lactic acid during cerebral ischemia, increase the ATP content, increase thedegree of oxygen dissociation in hemoglobin; Increase the resistance capacity for cerebral anoxia and occurring of spasm caused by cerebral ischemia, inhibit the generation of lipid peroxide in the brain. Oral absorption effectively, reach peak at 1 h, then metabolize into Vinpocetine in the body. The half-life of plasma elimination is about 1 hour. For 4 weeks in a row, no accumulation in the body. Clinical used in cerebral infarction sequela, cerebral hemorrhage sequelae, cerebral arteriosclerosis, cerebral vasospasm, brain endarteritis caused vertigo, tinnitus, headache, dizziness, limb numbness, incontinence and other clinical manifestations, depression, anxiety, sleep disorder and other mental symptoms. Clinical experience has shown that it is effective regardless of the length of the course of the disease, and the symptom is fixed or not. | [Indications]
1.Neurology: all kinds of cerebrovascular disease and its sequelae.
2.Cardiology: coronary heart disease, hardening of the arteries and blood clotting abnormalities, etc.
3.Eye: all kinds of views of visual impairment caused by poor circulation, etc.
4.Otolaryngology: hearing loss, tinnitus, vestibular dysfunction, etc.
5.Neurosurgery: all kinds of craniocerebral surgery back function rehabilitation. | [Side Effects]
Nervous system: head heavy, dizziness, occasional tiredness and side limb numbness, etc.
Digestive system: nausea, vomiting, loss of appetite, abdominal pain, diarrhea, etc.
Circulatory system: facial blushing, dizziness and other symptoms.
Blood system: white blood cells reduction.
Liver reaction: AST, ALT elevations, rare elevation of alkaline phosphatase.
Kidney reaction: blood urea nitrogen increasing.
Sometimes allergic reactions like skin rash, urticarial and pruritus may appear, then drug should be discontinued. Occasional mild lowering blood pressure, tachycardia, etc. | [Chemical Properties]
White crystal powder, odourless and tasteless. Soluble in chloroform or 96% ethanol, insoluble in water. The melting point is 147-147 oC (decomposition). [α]D20+114°(C=1��,pyridine). UV maximum absorption (96% ethanol): 229,275,315 nm (ε28200120, 00710). Acute toxicity LD50 in mice and rats (mg/kg): 534503 oral; 240133.8 intraperitoneal injection; 58.7, 42.6 intravenous injection. | [Usage]
Cerebrovascular drug. A alkaloid extracted from apocynaceae plant, Vincamine derivatives. Selectively inhibit vascular smooth muscle calcium dependency phosphodiesterase, and increase the content of cGMP, expanse cerebral vascular, which in turn increase cerebral blood flow, improve cerebral circulation, but has little effects on the cardiovascular and blood pressure. Effectively, good tolerance, less adverse reaction. Used for dizziness, headache, memory disorders, movement disorder, aphasia, hypertensive encephalopathy, etc.. Can also be used in brain blood circulation obstacle caused by mental or neurological symptoms.
This information is edited by ChemicalBook Xiao Nan. | [Production Method]
Vincamine extracted from small periwinkle (Vinca mino) of apocynaceae plant as raw material, dehydration to Apovincamine, then hydrolysis to Apovincaminic acid. Dissolved the acid (1.0 g, 0.003 mo1) and 1.0 g of potassium hydroxide in 80 ml of drying ethanol, add bromine ethane (0.4 g, 0.0036 mol), reflux 3 h. After the completion of reaction, cooling, evaporation to dry. Dissolve the leftovers in 500 ml of 2% sulfuric acid, and adjust the Ph to 8. Extracted with methylene chloride, drying with potassium carbonate, after the majority of methylene chloride has been evaporated, add in ethanol. Be placed overnight At 0 oC, filter the collected precipitation crystallization, washing with cold ethanol, drying, 0.66 g of Vinpocetine could be obtained.
Tabersonine extracted from apocynaceae plant willow small licorice leaf or periwinkle seed can also be as raw material, through multi-step synthesis.
| [Pharmacological Study]
Vinpocetine is a synthetic ethyl ester of apovincamine.
Vinpocetine has been used for cognitive impairment, but
the mechanism of action is unclear. A Cochrane review
(Szatmari and Whitehouse, 2003) of three short-term studies
involving 583 patients with dementia (AD, VaD, mixed)
concluded that patients treated with vinpocetine (30–60 mg/
day) showed modest bene�t compared to placebo. |
|
|