| Identification | More | [Name]
(R)-(+)-1-(4-Methylphenyl)ethylamine | [CAS]
4187-38-6 | [Synonyms]
(R)-(+)-1-(4-METHYLPHENYL)ETHYLAMINE
(R)-1-(4-METHYLPHENYL)ETHYLAMINE
(R)-1-P-TOLYLETHANAMINE
(R)-(+)-1-(P-TOLYL)ETHYLAMINE
(R)-(+)-4-(1-AMINOETHYL)TOLUENE
(R)-4-METHYL-ALPHA-METHYLBENZYLAMINE
(R)-(+)-ALPHA,4-DIMETHYLBENZYLAMINE
(R)-(+)-A-(P-TOLYL)ETHYLAMINE
(R)-alpha,p-Dimethylbenzylamine
Tolylethylamine
Methyl phenyl-ethylamine
(R)-(+)-ALPHA,4-DIMETHYLBENZYLAMINE, 98+ % (98% EE/GLC)
(R)-(+)-α,4-DIMETHYLBENZYLAMINE
(R)-(+)-1-(4-METHYLPHENYL)ETHYLAMINE , EE 98%
(R)-(+)-1-(P-TOLYL)ETHYLAMINE 98+%
(R)-(+)-1-(4-METHYLPHENYL)ETHYLAMINE, CHIPROS 98+%, EE 98%
Benzenemethanamine, α,4-dimethyl-, (αR)-
alpha,4-dimethylbenzylamine
(+)-p,α-Dimethylbenzylamine
(αR)-α,4-Dimethylbenzenemethanamine | [EINECS(EC#)]
624-182-1 | [Molecular Formula]
C9H13N | [MDL Number]
MFCD00145202 | [Molecular Weight]
135.21 | [MOL File]
4187-38-6.mol |
| Chemical Properties | Back Directory | [Appearance]
Colorless to light yellow liqui | [Melting point ]
<-20°C | [alpha ]
37 º (NEAT) | [Boiling point ]
205 °C (lit.) | [density ]
0.919 g/mL at 25 °C(lit.)
| [refractive index ]
n20/D 1.521(lit.)
| [Fp ]
180 °F
| [storage temp. ]
Inert atmosphere,2-8°C | [form ]
liquid | [pka]
9.20±0.10(Predicted) | [Specific Gravity]
0.919 | [optical activity]
[α]20/D +37°, neat | [Sensitive ]
Air Sensitive | [BRN ]
3195425 | [InChI]
InChI=1/C9H13N/c1-7-3-5-9(6-4-7)8(2)10/h3-6,8H,10H2,1-2H3/t8-/s3 | [InChIKey]
UZDDXUMOXKDXNE-SBYBRXNCNA-N | [SMILES]
[C@@H](C1C=CC(C)=CC=1)(N)C |&1:0,r| | [CAS DataBase Reference]
4187-38-6(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
C | [Risk Statements ]
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed .
R34:Causes burns. | [Safety Statements ]
S16:Keep away from sources of ignition-No smoking .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S27:Take off immediately all contaminated clothing .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) . | [RIDADR ]
UN 2619 8/PG 2
| [WGK Germany ]
3
| [HazardClass ]
8 | [PackingGroup ]
III | [HS Code ]
2921490090 |
| Hazard Information | Back Directory | [Chemical Properties]
Colorless to light yellow liqui | [Uses]
(R)-(+)-α,4-Dimethylbenzylamine can be used as a chiral salt of keto acid, employed in the solid-state photolysis studies of α-mesitylacetophenone derivatives. | [Application]
(R)-(+)-α,4-Dimethylbenzylamine reacts with
1,5-difluoro-2,4-dinitrobenzene (DFDNB) to form a chiral derivative
reagent(CDR) via substitution of one fluorine atom. | [General Description]
(R)-(+)-α,4-Dimethylbenzylamine(4187-38-6) is a chiral amine.
| [Synthesis]
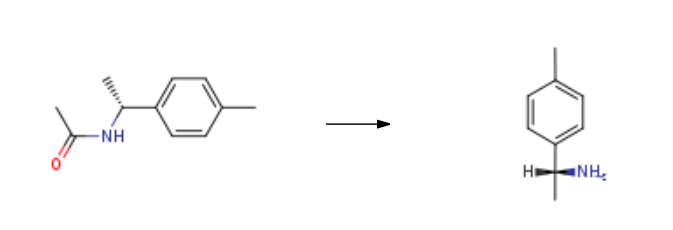
(R)-(+)-1-(4-Methylphenyl)ethylamine prepared from (R)-N-[1-(4-methylphenyl)ethyl]acetamide. The steps are as follows:
1 g of the compound obtained in the previous stage will be used. 2 g of n-butanol was added, 0.63 g of potassium hydroxide was added, and the temperature was raised to 100 ° C. After 24 hours of heat preservation, cool down to 10 to 20 ℃.After adding water and stirring for 0.5 hour, the mixture was allowed to stand for separation, and the organic layer was concentrated to n-butanol, and then subjected to vacuum distillation (80-100 ℃ packed column) to obtain (R)-1-(4-methylphenyl)ethylamine 0.63 g. The yield was 82%, the HPLC purity was 96.3%, and the enantiomer was 0.8%.
 | [structure and hydrogen bonding]
(R)-(+)-1-(4-Methylphenyl)ethylamine is a chiral molecule studied in the
context of crystallographic analysis and optimization. Through X-ray
diffraction analysis, the crystal structure of (R)-(+)-1-(4-Methylphenyl)ethylamine was determined, revealing the presence
of a significant hydrogen bond between the amine group and one of the
carbonyl groups. This hydrogen bond plays a crucial role in stabilizing
the (R)-(+)-1-(4-Methylphenyl)ethylamine molecule, which is a recurring
pattern observed in structurally similar compounds. Furthermore, the
(S)-(+)-enantiomer of (R)-(+)-1-(4-Methylphenyl)ethylamine was synthesized
using an enantiopure precursor, establishing an efficient method to
obtain this desired form of the molecule. |
|
|