| Identification | More | [Name]
Tosyl isocyanate | [CAS]
4083-64-1 | [Synonyms]
4-METHYLBENZENE-1-SULFONYL ISOCYANATE
4-TOLUENESULFONYL ISOCYANATE
P-TOLUENESULFONYL ISOCYANATE
P-TOLUENESULPHONYL ISOCYANATE
PTSI
TOLUENE-4-SULFONYL ISOCYANATE
TOSYL ISOCYANATE
4-Isocyanatosulphonyltoluene,
4-methyl-benzenesulfonylisocyanat
4-methyl-Benzenesulfonylisocyanate
4-Methylphenylsulfonyl isocyanate
Benzenesulfonyl isocyanate, 4-methyl-
Benzenesulfonylisocyanate,4-methyl-
p-Methylphenylsulfonyl isocyanate
p-Toluenesulfonic acid, anhydride with isocyanic acid
p-Toluensulfonyl isocyanate
p-Tolylsulfonyl isocyanate
p-Tosyl isocyanate
Sulfone, isocyanato tolyl
P-toluenesulfony6l isocyanate | [EINECS(EC#)]
223-810-8 | [Molecular Formula]
C8H7NO3S | [MDL Number]
MFCD00002030 | [Molecular Weight]
197.21 | [MOL File]
4083-64-1.mol |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R14:Reacts violently with water.
R36/37/38:Irritating to eyes, respiratory system and skin .
R42:May cause sensitization by inhalation. | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S28:After contact with skin, wash immediately with plenty of ... (to be specified by the manufacturer) .
S30:Never add water to this product . | [RIDADR ]
UN 2206 6.1/PG 3
| [WGK Germany ]
1
| [RTECS ]
DB9032000 | [F ]
10 | [TSCA ]
Yes | [HazardClass ]
6.1 | [PackingGroup ]
III | [HS Code ]
29309090 |
| Hazard Information | Back Directory | [Hazard]
Moderately toxic by ingestion. Low toxicity by inhalation. A mild skin and moderate eye
irritant. | [Chemical Properties]
clear liquid | [Uses]
p-Toluenesulfonyl isocyanate is a reagent used to prepare acetylated syn-1,2-diols,1 oxazolidin-2-ones,2 2,3-diamino acids,3 and N-tosylcarbonamides.4
| [Synthesis Reference(s)]
Tetrahedron Letters, 35, p. 9609, 1994 DOI: 10.1016/0040-4039(94)88523-0 | [General Description]
p-Toluenesulfonyl isocyanate undergoes palladium-catalyzed bis-allylation reaction with allylstannanes and allyl chlorides. | [Reactivity Profile]
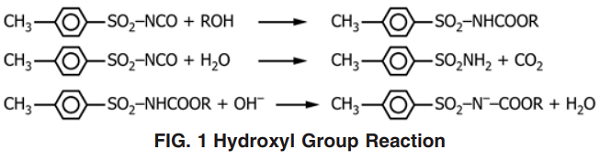
p‐Toluenesulfonyl Isocyanate (PTSI) is recommended especially for one‐component, low‐VOC polyurethane coatings. The reaction of PTSI with water introduced from pigments and solvents in the paint formulation generates carbon dioxide and soluble inert chemical products. Experience has demonstrated that 13 grams of PTSI effectively scavenges 1 gram of water. PTSI reacts with water in a 1 to 1 molar ratio, generating PTSA and carbon dioxide gas. This reaction occurs readily at room temperature and does not require heating. The PTSA generated in the process is essentially an inert material that does not further react with PTSI or other isocyanate groups, resulting in the formation of insoluble ureas. The hydroxyl group is reacted with excess p-toluenesulfonyl isocyanate (TSI) to form an acidic carbamate. Water is added to convert unreacted isocyanate to sulfonamide, followed by direct potentiometric titration of the acidic carbamate with tetrabutylammonium hydroxide (Bu4NOH) in nonaqueous medium.
| [Safety]
Danger! Harmful if swallowed, inhaled or absorbed through the skin. Causes skin and severe eye irritation. It may cause an allergic respiratory response. Reacts spontaneously and violently with water, alcohols, amines, acids and alkaline solutions. These substances should not be poured into a vessel containing PTSI. Prolonged exposure of PTSI to elevated temperatures can result in violent decomposition. Temperatures above 170?C are to be avoided. Reaction with water results in the generation of carbon dioxide. Reaction vessels should be vented to avoid pressure build‐up. Vent through a calcium chloride tube to prevent moisture from entering vessels. Do not get in the eyes, on the skin, or clothing. Avoid breathing vapour or mist. Keep the container closed. Use with adequate ventilation. Wash thoroughly after handling. If handled indoors, provide adequate mechanical exhaust ventilation or wear NIOSH/MSHA approved air ‐line respirator or SCBA. Wear impervious PVC or rubber gloves, goggles, and protective clothing. PTSI must be stored in air‐tight containers. | [Synthesis]
Add the organic azide (0.75 mmol), Pd(OAc)2
(5.6 mg, 5 mol%) to an oven-dried Schlenk tube (10 mL). Purge the tube
and backfill with CO (three cycles) from a balloon. Inject anhydrous
MeCN (3.0 mL) into the tube. Stir at 80??C for 4 h under CO atmosphere
(balloon). Concentrate the mixture under reduced pressure. Purify the
residue by column chromatography (petroleum ether/EtOAc 3:1.-.1:1).

|
|
|