| Identification | More | [Name]
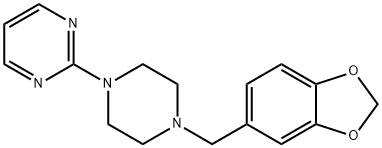
2-[4-(1,3-Benzodioxol-5-ylmethyl)piperazin-1-yl]pyrimidine | [CAS]
3605-01-4 | [Synonyms]
2-[4-(1,3-benzodioxol-5-ylmethyl)piperazin-1-yl]pyrimidine
PIRIBEDIL
Piribendyl
PIRIBEDIL(FORR&DONLY0
PIRIBEDIOL
ET-495
EU-4200
Trivastal
Trivastan | [EINECS(EC#)]
222-764-6 | [Molecular Formula]
C16H18N4O2 | [MDL Number]
MFCD00868264 | [Molecular Weight]
298.34 | [MOL File]
3605-01-4.mol |
| Chemical Properties | Back Directory | [Melting point ]
98° | [Boiling point ]
439.76°C (rough estimate) | [density ]
1.1183 (rough estimate) | [refractive index ]
1.6000 (estimate) | [storage temp. ]
2-8°C | [solubility ]
Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly) | [form ]
Solid | [pka]
7.17±0.10(Predicted) | [color ]
White | [Merck ]
14,7493 | [CAS DataBase Reference]
3605-01-4(CAS DataBase Reference) |
| Safety Data | Back Directory | [RTECS ]
UV9704000 | [HS Code ]
2933.59.8000 | [Toxicity]
LD50 in mice (mg/kg): 88 i.v., 690 i.p., 1460 orally (Laubie) |
| Hazard Information | Back Directory | [Chemical Properties]
White Solid | [Originator]
Trivastal, Eutherapie ,France,1969 | [Uses]
Piribedil is an antiparkisonian agent that acts as a dopamine agonist. Piribedil also displays α2-adrenergic antagonist properties. Piribedil has also been shown to counteract age-related memory impai
rment by improving memory and attention as well as increasing the velocity of psychomotor reactions and lability of nervous processes. | [Uses]
Piribedil is an antiparkisonian agent that acts as a dopamine agonist. Piribedil also displays α2-adrenergic antagonist properties. Piribedil has also been shown to counteract age-related memory impairment by improving memory and attention as well as increasing the velocity of psychomotor reactions and lability of nervous processes. | [Definition]
ChEBI: 2-[4-(1,3-benzodioxol-5-ylmethyl)-1-piperazinyl]pyrimidine is a N-arylpiperazine. | [Manufacturing Process]
To a solution of 21 g of 1-(3':4'-methylenedioxybenzyl)-piperazine in solution in 300 cc of anhydrous xylene there were added 28 g of anhydrous potassium carbonate and then 11.3 g of 2-chloropyrimidine. The suspension was then heated for 9 hours at boiling point (130°C). After this time, the mixture was cooled and extracted several times with 10% hydrochloric acid. The acid solution obtained was washed with ether and then rendered alkaline with potassium carbonate; the oily product which was separated was extracted with chloroform and this, after drying with potassium carbonate and evaporation, gave an oily residue weighing 20 g. By dissolution in boiling ethanol and crystallization, 15 g of crystals melting at 96°C were recovered. | [Therapeutic Function]
Vasodilator |
|
|