| Identification | More | [Name]
SAMARIUM(II) IODIDE | [CAS]
32248-43-4 | [Synonyms]
SAMARIUM(II) IODIDE
SAMARIUM IODIDE
SAMARIUM(II) IODIDE SOLUTION, STAB., ~0. 1 M IN THF
SAMARIUM(II) IODIDE, 0.1M SOLUTION IN TE TRAHYDROFURAN
SAMARIUM(II) IODIDE, POWDER, 99.9+%
SAMARIUM (II) IODIDE 0.1M IN THF
0.1MinTHF,stabilized
Samarium(II)iodide,0.1MinTHF,stab.
Samarium iodide (SmI2)
samarium(ii) iodide solution
Samarium(II) iodide, 0.07-0.12M in THF, stab.
Samarium(II) Iodide (ca. 0.1mol/L in Tetrahydrofuran)
Diiodosamarium(II)
Samarium(II) diiodide | [EINECS(EC#)]
203-726-8 | [Molecular Formula]
I2Sm | [MDL Number]
MFCD00058873 | [Molecular Weight]
404.17 | [MOL File]
32248-43-4.mol |
| Chemical Properties | Back Directory | [Appearance]
Deep bleu-green solution | [Melting point ]
526 ±3° | [Boiling point ]
solid: 1580℃ [CRC10] | [density ]
0.922 g/mL at 25 °C
| [Fp ]
−2 °F
| [storage temp. ]
2-8°C | [solubility ]
reacts with H2O | [form ]
powder
| [color ]
Deep blue-green | [Sensitive ]
Air & Moisture Sensitive | [Merck ]
8349 | [Exposure limits]
ACGIH: TWA 50 ppm; STEL 100 ppm (Skin)
OSHA: TWA 200 ppm(590 mg/m3)
NIOSH: IDLH 2000 ppm; TWA 200 ppm(590 mg/m3); STEL 250 ppm(735 mg/m3) | [InChIKey]
UAWABSHMGXMCRK-UHFFFAOYSA-L | [CAS DataBase Reference]
32248-43-4(CAS DataBase Reference) |
| Hazard Information | Back Directory | [Chemical Properties]
Deep bleu-green solution | [Uses]
In organic synthesis and electron transfer reactions; in discharge lamps. | [Description]
Samarium(II) iodide, SmI2, when employed as a solution for organic synthesis, is known as “Kagan’s reagent.” SmI2 is a green solid and its solutions are green as well. It is a strong one-electron reducing agent that is used in organic synthesis. Solid, solvent-free SmI2 forms by high-temperature decomposition of samarium(III) iodide (SmI3). | [Purification Methods]
A possible impurity is SmI3 from which it is made. If present, grind the solid to a powder and heat it in a stream of pure H2. The temperature (~ 500-600o) should be below the m (~ 628o) of SmI3, since the molten compounds react very slowly. [Wetzel in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol II pp 1149, 1150 1965.] | [reaction suitability]
reagent type: catalyst
core: samarium
reagent type: reductant |
| Safety Data | Back Directory | [Hazard Codes ]
F,Xi | [Risk Statements ]
R11:Highly Flammable.
R19:May form explosive peroxides.
R36/37:Irritating to eyes and respiratory system . | [Safety Statements ]
S16:Keep away from sources of ignition-No smoking .
S29:Do not empty into drains .
S33:Take precautionary measures against static discharges . | [RIDADR ]
UN 2056 3/PG 2
| [WGK Germany ]
3
| [F ]
1-10 | [HazardClass ]
3 | [PackingGroup ]
II | [HS Code ]
28276000 |
| Questions And Answer | Back Directory | [Reaction]
SmI2 has been used extensively in literature due to its large reduction potential. Known as a single-electron transfer reagent.
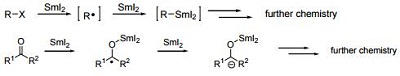
| [Mechanism of SmI2-mediated Reactions]
Radicals and anions from organohalides
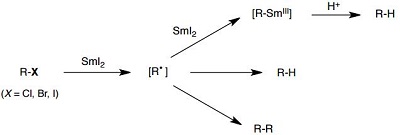
Reduction of halides: iodides > bromides > chloride
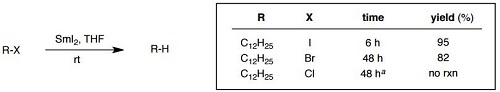
reaction carried out at 60 °C |
|
|