| Identification | More | [Name]
Sodium cyanoborohydride | [CAS]
25895-60-7 | [Synonyms]
SODIUM CYANOBOROHYDRIDE
SODIUM CYANOTRIHYDRIDOBORATE
SODIUM CYANOTRIHYDROBORATE
(cyano-C)trihydro-,sodium,(T-4)-Borate(1-)
sodium,(beta-4)-borate(1-(cyano-c)trihydro-
Sodium cyonobrohydriole
SODIUM CYANOBOROHYDRIDE, 5.0M SOLUTION I N AQUEOUS CA. 1M SODIUM HYDROXIDE
SODIUM CYANOBOROHYDRIDE, 1.0M SOLUTION I N TETRAHYDROFURAN
Sodiumcyanoborohydride,95%
Borate(1-), (cyano-.kappa.C)trihydro-, sodium, (T-4)-
sodium cyanoborohydride solution
Sodium Cyanoborohydride [Reducing Agent]
Sodium cyanotrihydridoborate, typically 95%
Borate(1-), (cyano-kC)trihydro-, sodium, (T-4)-
Sodium borocyanohydride
Sodium cyanotrihydroborate(1-) | [EINECS(EC#)]
247-317-2 | [Molecular Formula]
CH3BNNa | [MDL Number]
MFCD00003516 | [Molecular Weight]
62.84 | [MOL File]
25895-60-7.mol |
| Chemical Properties | Back Directory | [Appearance]
White solid | [Melting point ]
>242 °C (dec.) (lit.) | [Boiling point ]
307°C | [density ]
1.083 g/mL at 25 °C
| [Fp ]
−1 °F
| [storage temp. ]
Store under Argon | [solubility ]
H2O: may be clear to slightly hazy
| [form ]
Powder | [color ]
White | [Specific Gravity]
1.2 | [Stability:]
Stable. Hygroscopic. Reacts violently with water, giving off and igniting hydrogen. Do not use water on fires involving this chemical-instead use dry soda ash. Incompatible with strong acids, water, strong oxidizing agents. | [biological source]
synthetic | [Water Solubility ]
2120 g/L at 29 ºC (dec.) | [Sensitive ]
Moisture Sensitive | [Merck ]
14,8606 | [BRN ]
4152656 | [CAS DataBase Reference]
25895-60-7(CAS DataBase Reference) | [Storage Precautions]
Store under nitrogen;Moisture sensitive | [EPA Substance Registry System]
25895-60-7(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
T+,N,T,F | [Risk Statements ]
R26/27/28:Very Toxic by inhalation, in contact with skin and if swallowed .
R32:Contact with acids liberates very toxic gas.
R34:Causes burns.
R50/53:Very Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment .
R16:Explosive when mixed with oxidizing substances.
R15:Contact with water liberates extremely flammable gases.
R11:Highly Flammable.
R51/53:Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment .
R36:Irritating to the eyes.
R23/24/25:Toxic by inhalation, in contact with skin and if swallowed .
R19:May form explosive peroxides.
R14:Reacts violently with water. | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S28:After contact with skin, wash immediately with plenty of ... (to be specified by the manufacturer) .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S60:This material and/or its container must be disposed of as hazardous waste .
S61:Avoid release to the environment. Refer to special instructions safety data sheet .
S8:Keep container dry .
S43:In case of fire, use ... (indicate in the space the precise type of fire-fighting equipment. If water increases the risk add-Never use water) .
S1:Keep locked up .
S16:Keep away from sources of ignition-No smoking . | [RIDADR ]
UN 3179 4.1/PG 2
| [WGK Germany ]
1
| [F ]
10-21 | [Hazard Note ]
Toxic/Highly Flammable | [TSCA ]
Yes | [HazardClass ]
6.1 | [PackingGroup ]
I | [HS Code ]
28500020 |
| Hazard Information | Back Directory | [Description]
Sodium cyanoborohydride (NaBH3CN) is a selective reducing agent used for a variety of chemical reductions, including aldehyde, ketones, acetals, epoxides, oximes, enamines, reductive aminations of aldehydes and ketones, and reductive alkylations of amines and hydrazines. The utility of sodium cyanoborohydride as a reducing agent is greatly enhanced by its stability under acid conditions, and its solubility in aprotic solvents. Sodium cyanoborohydride is a milder and more selective reducing agent than sodium borohydride.
Sodium cyanoborohydride is a weaker and more-selective reducing agent than sodium borohydride because of the electron-withdrawing effect of the cyano group. It has the further advantage that it is stable in acid to pH = 3 and can be employed to effect reductions in the presence of functional groups that are sensitive to the more-basic conditions of reduction with sodium borohydride.
Aldehydes and ketones are unaffected by sodium cyanoborohydride in neutral solution, but they are readily reduced to the corresponding alcohol at pH=3-4 by way of the protonated carbonyl group. By previous exchange of the hydrogens of the borohydride for deuterium or tritium, by reaction with D2O or tritiated water, an efficient and economical route is available for deuteride or tritiide reduction of aldehydes and ketones.
| [Chemical Properties]
Sodium cyanoborohydride is a white or slightly yellow solid powder, It is a versatile reducing agent stable in aqueous solution at pH 7.2. It is a weaker reducing agent as compared to NaBH4. It effectively reduces Schiff bases. NaCNBH4 participates in the reductive methylation for the efficient and selective conversion of the e-amino groups of lysyl residues in proteins to the mono- and dimethyl derivatives. | [Physical properties]
Sodium cyanotrihydridoborate, sodium cyanoborohydride, sodium cyanoboranate, NaBH3CN, is a white, amorphous powder, melting with decomposition at 240 – 242℃. Contact with the atmosphere must be avoided as it is extremely hygroscopic. It is very soluble in polar, especially protic, solvents such as water, alcohols, amines, and THF, but not in diethyl ether or hydrocarbons. | [Uses]
Sodium Cyanoborohydride is a commonly used as a reagent in the reductive amination of aldehydes and ketones and in the reductive alkylation of amines. It is also used in the synthesis of a novel phenolate-bridged dilanthanum(III) complex of interest as a model for metalloproteins as well as for its importance in basic and applied chemistry. | [Preparation]
Synthesis of Sodium cyanoborohydride(NaBH3CN): NaBH3CN was made by stirring equimolar BH3·THF (~1 M) with NaCN in THF in excellent yield.To a rapidly stirred slurry of sodium borohydride (80.2g, 2.09 mol) in THF (1000mL) in a 2-L flask is added a solution of hydrogen cyanide in THF (294g containing 58.8g of hydrogen cyanide) at 25°C. Evolution of hydrogen occurs slowly during the addition. Following the addition, the reaction mixture is stirred for 1 h at 25°C and then heated at reflux until hydrogen evolution has ceased. Filtration followed by vacuum removal of THF gives white solid sodium cyanoborohydride; yield 120g (91%). | [Reactions]
Sodium cyanoborohydride are frequently used for reductive aminations. Since the reaction rate for the reduction of iminium ions is much faster than for ketones or even aldehydes, the reductive amination can be carried out as a one-pot procedure by introducing the reducing agent into a mixture of the amine and carbonyl compound.
Contact with strong acids liberates the highly toxic gas HCN. A safer reducing agent with comparable reactivity is sodium triacetoxyborohydride. Reduction with Sodium Cyanoborohydride:
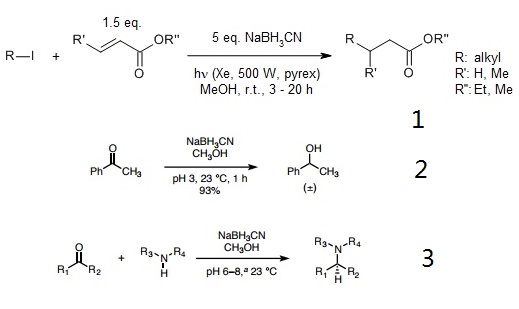
Tin-free Giese reaction of alkyl iodides with electron-deficient alkenes and the related radical carbonylation process proceeded efficiently in the presence of sodium cyanoborohydride and tetrabutylammonium cyanoborohydride. Transfer of iodine followed by hydride reduction of the resulting carbon-iodine bond is proposed as a possible mechanism.
Borch and co-workers showed that sodium cyanoborohydride and lithium cyanoborohydride are acid-stable reagents capable of rapidly reducing carbonyl compounds to alcohols at pH 3-4, presumably via a protonated carbonyl cation.
With care to maintain a pH of 6-7, a mixture of a ketone or aldehyde reactant, an amine, and sodium cyanohydride provides products of reductive amination selectively, without competitive reduction of the carbonyl substrate. Though the conditions of the Borch reduction are mild, sodium cyanoborohydride is highly toxic, as are its byproducts. The pH was maintained by addition of HCl and/or KOH as needed using bromocresol green as an indicator.
 | [Hazard]
Flammable solid. Dust may form explosive mixture with air. Fire-water run-off in sewers may
create fire or explosion hazard. This product is toxic. Severe corrosive hazard. Water used for
fire extinguishing, which has been in contact with the product, may be corrosive. | [reaction suitability]
reagent type: reductant | [Chemical Reactivity]
Sodium cyanoborohydride is used as a selective reducing agent in the synthesis of pharmaceuticals. Its special reducing properties arise from its remarkable resistance to hydrolysis. It is stable even at pH 3 in water and methanol, its rate of hydrolysis being slower than that of NaBH4 by a factor of 108. Reactivity and stereoselectivity are considerably affected by the nature and acidity of the solvent. In polar aprotic solvents such as hexamethylphosphoric triamide (HMPT) or dimethyl sulfoxide (DMSO) it reduces aliphatic halides and toluenesulfonates to the corresponding hydrocarbons. Under these conditions other reducible functional groups such as epoxides, ketones, and aldehydes are unaffected. | [Purification Methods]
Sodium cyanoborohydride (10g) is dissolved in THF (80mL) and 1 M methanolic hydrochloric acid is added until pH reaches 9. The solution is then poured with stirring into dioxan (250mL). The precipitate is collected and stirred for 2 h in ethyl acetate (250mL). This solution is filtered, heated to reflux on a steam bath and then dioxan (150mL) is added slowly with swirling. This solution is slowly cooled to room temperature, chilled and filtered. The crystalline dioxan complex is dried in vacuo for 4 h at room temperature, then for 4 h at 80° C; yield 6.74g, purity >98% sodium cyanoborohydride by iodometric titration. |
|
|