| Identification | More | [Name]
2,3,5,6-Tetrachloropyridine | [CAS]
2402-79-1 | [Synonyms]
2,3,5,6-TETRACHLOROPYRIDINE
Tetrapyridine
Pyridine, 2,3,5,6-tetrachloro-
2,3,5,6-Tetrachloropyridine 98+%
2,3,5,6-tetrachloro-pyridin | [EINECS(EC#)]
219-283-9 | [Molecular Formula]
C5HCl4N | [MDL Number]
MFCD00092428 | [Molecular Weight]
216.88 | [MOL File]
2402-79-1.mol |
| Chemical Properties | Back Directory | [Melting point ]
90.5°C | [Boiling point ]
251.6°C | [density ]
2.0309 (rough estimate) | [vapor pressure ]
3Pa at 25℃ | [refractive index ]
1.6000 (estimate) | [Fp ]
188°C | [storage temp. ]
Inert atmosphere,Room Temperature | [solubility ]
very soluble in Ether,Ethanol | [form ]
Crystalline | [pka]
-5.50±0.50(Predicted) | [color ]
White to Almost white | [BRN ]
129639 | [InChIKey]
FATBKZJZAHWCSL-UHFFFAOYSA-N | [LogP]
3.87 at 30℃ and pH7 | [Surface tension]
72.4mN/m at 20.7mg/L and 21.3℃ | [CAS DataBase Reference]
2402-79-1(CAS DataBase Reference) | [EPA Substance Registry System]
2402-79-1(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed .
R36/37/38:Irritating to eyes, respiratory system and skin .
R51/53:Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S60:This material and/or its container must be disposed of as hazardous waste .
S61:Avoid release to the environment. Refer to special instructions safety data sheet .
S36:Wear suitable protective clothing . | [RIDADR ]
2811 | [RTECS ]
UT8225000 | [Hazard Note ]
Harmful/Irritant | [TSCA ]
Yes | [HazardClass ]
6.1 | [PackingGroup ]
III | [HS Code ]
2933392000 | [Hazardous Substances Data]
2402-79-1(Hazardous Substances Data) | [Toxicity]
mouse,LD50,intraperitoneal,1150mg/kg (1150mg/kg),BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY)LIVER: FATTY LIVER DEGERATIONBEHAVIORAL: ANTIPSYCHOTIC,Toxicology and Applied Pharmacology. Vol. 11, Pg. 361, 1967. |
| Hazard Information | Back Directory | [Uses]
2,3,5,6-Tetrachloropyridine is used as a chemical intermediate in the production of chlorpyrifos and triclopyr. | [Synthesis]
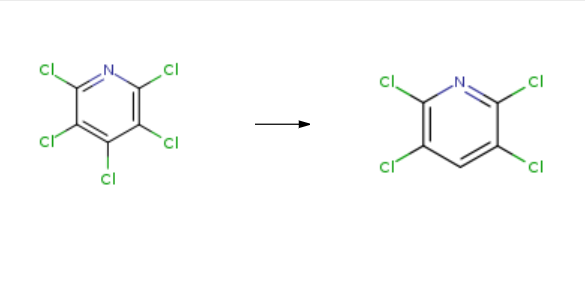
2,3,5,6-Tetrachloropyridine is synthesised in three steps by chemical reaction using 2,3,4,5,6-pentachloropyridine as a raw material. The specific synthesis steps are as follows:
Step (1): 2 parts of pentachloropyridine was dissolved in 200 parts of ethanol at 40 ℃ until 0.6 part of metallic manganese powder was added after the raw material was completely dissolved; 1.3 parts of ammonium acetate, 5 parts of water and 10 parts of ethanol were mixed Uniform dropping, dropping time of 2h; After completion of the dropwise addition, the reaction solution was stirred for 4 hours.
Step (2): add ammonia to adjust the pH value of 8~9, heated to 65 , through 3L / min air 1h reaction was oxidized.
Step (3): Oxidation liquid filtration: The resulting solid was filtered and separated by washing with 20 parts of toluene. Ethanol washed twice, washed twice, after dryingproduct. Products tested by the magnetic content of manganese oxide products, the specific surface area of 53m2 / g, Mn content of 70.90%, CaO content0.0012%, K content 0.0005%, Na content 0.0025%, S content 0.0070%. The XRD pattern of manganese tetroxide and the comparison with the standard map is Mn3O44. Figure 5 (a) and (b) are scanning electron micrographs. It can be seen that the product is a hollow spherical structure. (C) and (d) are transmission electron micrographs, and the hollow structure can be seen and the product is from 10 to 25 nm Grain composition. The filtrate was filtered and the organic solution of the solution of manganese tetrachloride was combined and evaporated to recover the toluene solvent. Then, 10 parts of 5% dilute hydrochloric acid was added at a temperature of 65 ℃ and stirred for 1 h to obtain 2,3,5,6-tetrachloropyridine ; The yield was 99.5%.
 |
|
|