| Identification | More | [Name]
3,4-Dimethoxyphenol | [CAS]
2033-89-8 | [Synonyms]
3,4-DIMETHOXYPHENOL
4-HYDROXYVERATROLE
3,4-dimethoxy-pheno
Phenol, 3,4-dimethoxy- | [EINECS(EC#)]
217-995-4 | [Molecular Formula]
C8H10O3 | [MDL Number]
MFCD00008390 | [Molecular Weight]
154.16 | [MOL File]
2033-89-8.mol |
| Chemical Properties | Back Directory | [Appearance]
Red Crystal Powder | [Melting point ]
79-82 °C (lit.) | [Boiling point ]
167°C/14mm | [density ]
1.1690 (rough estimate) | [refractive index ]
1.4740 (estimate) | [Fp ]
167°C/14mm | [storage temp. ]
Keep in dark place,Inert atmosphere,Room temperature | [solubility ]
Chloroform (Slightly), Methanol (Slightly) | [form ]
Fine Crystalline Powder | [pka]
10.08±0.18(Predicted) | [color ]
Off-white to light beige | [Water Solubility ]
Soluble in water. | [Sensitive ]
Light Sensitive | [BRN ]
1366650 | [LogP]
1.157 (est) | [CAS DataBase Reference]
2033-89-8(CAS DataBase Reference) | [NIST Chemistry Reference]
Phenol, 3,4-dimethoxy-(2033-89-8) | [EPA Substance Registry System]
2033-89-8(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing .
S37/39:Wear suitable gloves and eye/face protection . | [WGK Germany ]
3
| [F ]
10 | [TSCA ]
Yes | [HS Code ]
29095000 |
| Hazard Information | Back Directory | [Chemical Properties]
Red Crystal Powder | [Uses]
3,4-Dimethoxyphenol is used in the preparation of 5,6-dimethoxy benzofuranone derivatives and multi-target anti Alzheimer compounds. It is also used in the preparation of 3,4-dimethoxyphenyl-beta-D-glucopyranoside and 4-(but-2-enyloxy)-1,2-dimethoxybenzene. Further, it serves as a precursor for 4H-chromenes synthesis. | [Uses]
A substituted alkynyl phenoxy compound as new synergists in pesticidal compositions. | [Synthesis]
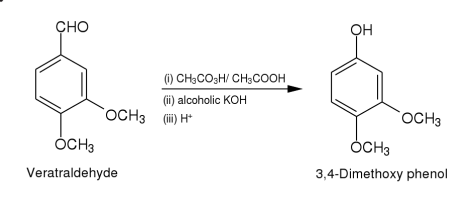
3,4-Dimethoxyphenol is synthesised using Veratraldehyde as a raw material by chemical reaction. The specific synthesis steps are as follows:
Method (i): To a solution of vertraldehyde (2.11)(5 g,0.03 mole)in glacial acetic
acid (30 ml)is added dropwise to a solution of peracetic acid5(15 ml)
during 30 min.The temperature of the reaction mixture rises,it is kept at
40-45 by cooling.The reaction mixture is left for 10 hr and then
concentrated to about 15 ml in vacuo.The residue is extracted with ether
(2 x 20 ml).The ether layer is distilled.The formate ester of 3,4-
dimethoxyphenol thus obtained is hydrolysed by refluxing with potassium
hydroxide (10 g)in aqueous alcohol (1:4,100 ml)for 1 hr.The reaction
mixture is concentrated in vacuo almost to dryness.It is dissolved in water
(20 ml),the solution rendered acidic with dilute sulphuric acid and
extracted with ether.The ether extract is dried (sodium sulphate)and
distilled.The oily residue thus obtained is subjected to column
chromatography over silica gel(2 x 30 cm).Elution with benzene gave 3,4-dimethoxyphenol as yellow solid.Yield 3g(65.2%).It is crystallised from
benzene as yellow crystalline needles.M.p.78-80(lit.m.p.78-80).
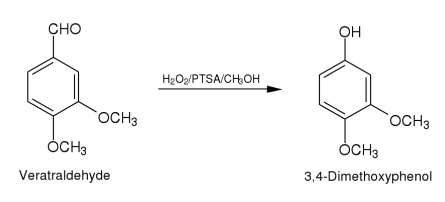
Method (ii): A mixture of veratraldehyde (2.11)(1.66 g,0.01 mole),hydrogen
peroxide (30%,2.6 ml,0.023 mole),p-toluenesulphonic acid (6.88 g,
0.036 mole)and methanol (5 ml)is stirred at room temperature.When the
reaction is complete (as monitored by TLC)the reaction mixture is diluted
with water (50 ml)and extracted with dichloromethane (5 x 50 ml).The
extract is dried (anhydrous sodium sulphate)and purified by column
chromatography over silica gel(2 x 30 cm).Elution with benzene gave 3,4.
dimethoxyphenol as an yellow solid.Yield 1.16 g (75.3%).M.p.78-80.
 | [storage]
4°C, protect from light |
|
|