| Identification | More | [Name]
Gatifloxacin hydrochloride | [CAS]
160738-57-8 | [Synonyms]
1-cyclopropyl-6-fluoro-8-methoxy-7-(3-methyl-piperazin-1-yl)-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid hydrochloride
gatifloxacin hydrochloride
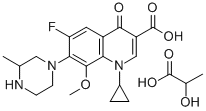
GATIFLOXACIN LACTATE
Gatifloxacin acid ester
Gatifloxacin base
4-dihydroquinoline-3-carboxylic acid
GatifloxacineHCL
1-Cyclopropyl-6-fluoro-1,4-dihydro-8-methoxy-7-(3-methylpiperazin-1-yl)-4-oxo-3-quinolinecarboxylicac
1-cyclopropyl-6-fluoro-8-methoxy-7-(3-methyl-piperazin-1-yl)-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid hydrochloride | [EINECS(EC#)]
1308068-626-2 | [Molecular Formula]
C22H28FN3O7 | [MDL Number]
MFCD08063913 | [Molecular Weight]
465.47 | [MOL File]
160738-57-8.mol |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S27:Take off immediately all contaminated clothing .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection . | [HS Code ]
29419000 |
| Hazard Information | Back Directory | [Description]
Gatifloxacin is a novel orally-active fluoroquinolone antibiotic with a
potent and broad spectrum of activity against gram-positive (S.pneumonia,
S.aureus) and gram-negative bacteria. It shows good activity against strains
resistant to established antituberculosis agents. Gatifloxacin was marketed for the treatment of respiratory tract and urinary infections, particularly the treatment
of certain community-acquired infections (such as bronchitis, pneumonia and
common sexually-transmitted diseases). Comparative studies with ciprofloxacin,
ofloxacin and sparfloxacin against fluoroquinolone-resistant organisms indicated
a better or equivalent effectiveness with less phototoxic adverse effects. | [Chemical Properties]
pale yellow prisms | [Originator]
Kyori (Japan) | [Brand name]
Tequin |
|
|